Superoxide Dismutase - Manual
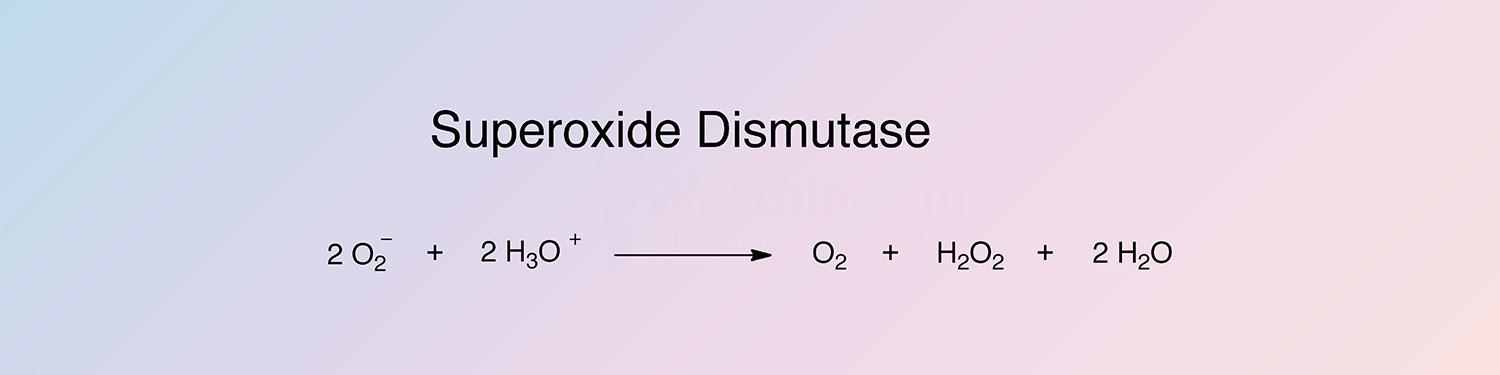
Superoxide dismutase (SOD) catalyzes the destruction of the O2- free radical.
It protects oxygen-metabolizing cells against harmful effects of superoxide free-radicals (Petkau et al. 1975; Fridovich 1972, 1973; Lavelle et al. 1973; Paschen and Weser 1973). It has been reviewed by Malmström et al. (1975).
McCord (1974) found that SOD protects hyaluronate against depolymerization by free-radicals and indicated that exogenous SOD might have an anti-inflammatory effect (Salin and McCord 1975). The O2- ion, which has been considered important in aging, lipid peroxidation and the peroxidative hemolysis of red blood cells (Fee and Teitelbaum 1972), is formed by the univalent reduction of O2 during various enzymatic reactions or by ionizing radiation. (See also Fee et al. 1975). There is also superoxide radical formation during leukocyte phagocytosis (Allen et al. 1974; DeChatelet et al. 1974). See also Dionisi et al. (1975). Winterbourn et al. (1975) indicate that SOD deficiency might lead to Heinz body hemolytic anemia. Fridovich (1986) reports on the biological effects of the superoxide radical.
Superoxide dismutase is widespread in nature. Gregory et al. (1974) indicate it to be present in all oxygen-metabolizing cells. Hewitt and Morris (1975) have found it in anaerobic bacteria. It has been purified from diverse sources such as: fungi (Rapp et al. 1973); green pea (Sawda et al. 1972); Streptococcus mutans (Vance et al. 1972); wheat germ (Beauchamp and Fridovich 1973); E. coli (Gregory et al. 1973); Saccharomyces cerevisiae (Goscin and Fridovich 1972) and Neurospora crassa (Misra and Fridovich 1972).
Three superoxide dismutases are characterized by different metal content. A blue-green Cu(II)-Zn(II) enzyme comes from human and bovine erythrocytes, a wine-red Mn(III) protein is found in E. coli, and in chicken, and rat (Peeters-Joris et al. 1975) liver mitochondria (Tyler 1975) and a yellow Fe(III) enzyme from E. coli (Villafranca et al. 1974). It is of interest that the chicken liver cytosomal enzyme is the copper-zinc type (Weisiger and Fridovich 1973). Peeters-Joris et al. (1975) show SOD activity in many organs of the rat. Gregory et al. (1973) have reported on intra-cellular sites and functions.
Bovine erythrocyte SOD, to which the following data apply, has been extensively studied. It is identical to the enzyme from human erythrocytes and from beef heart (Bannister et al. 1971; Keele et al. 1971 and Nyman 1960).
Characteristics of Superoxide Dismutase from Bovine Erythrocytes:
Superoxide dismutase consists of two subunits of identical molecular weight joined by a disulfide bond. The molecular weight is 32,500 (Keele et al. 1971). There are two Cu(II) and two Zn(II) atoms per molecule (Bannister et al. 1971). Crystal studies have been reported by Richardson et al. (1972) and Lieberman and Fee (1973). The amino acid sequence was determined by Steinman, Naik, Abernethy and Hill (1974), Abernethy et al. (1974) and Evans et al. (1974); the subunit tertiary structure by Richardson et al. (1975). Rotilio et al. (1972, 1973, 1974) have reported on the roles of copper and zinc. According to Forman and Fridovich (1973) zinc has a structural, stabilizing role, while Cu2+ is directly involved in the catalytic activity. See also Calabrese et al. (1991). Both zinc and copper have been removed to yield the apoenzyme (Carrico and Deutsch 1970). Fee and co-workers have published studies on the metal binding sites (Fee 1973; Fee and DiCorleto 1973; Fee and Gaber 1972). and enzymatic activity (Fee et al. 1973). Beem et al. (1974) report on the replacement of zinc by cobalt, mercury, and cadmium. Forman et al. (1973) and Rigo et al. (1975) indicate histidine to be involved at the active site. Nuclear magnetic resonance studies have been reported by Stokes et al. (1973), Lieberman and Fee (1973) and Villafranca et al. (1974). Superoxide dismutase activity and kinetics have been reported by Halliwell (1975), Rigo et al. (1975), Fielden et al. (1974), Goda et al. (1974), Michelson (1974), Hodgeson and Fridovich (1973), Rotilio (1973), and Grunow and Schöpp (1989).
32,500 (Keele et al. 1971).
4.95 (Bannister et al. 1971).
The protein is peculiar in that it does not show an absorption maximum at 280 nm (McCord and Fridovich 1969; Bannister et al. 1971). According to Symonyan and Nalbandyan (1972) A259/A680 = 30.
Cyanide inhibits cupro-zinc SOD but has no effect on the mangano enzyme of chicken liver mitochondria (Beauchamp , in Weisiger and Fridovich 1973). SOD is inactivated by H2O2 (Symonyan and Nalbandyan 1972, Fielden et al. 1973) and may be protected by catalase (Bray et al. 1974) with which it is usually associated. Hartz et al. (1973) found that in some tissue including cerebral cortex and thyroid, SOD is present but not catalase.
SOD is an unusually stable enzyme although its apoenzyme is very unstable. (Forman and Fridovich 1973). Worthington SOD retains its activity for up to a year at 5°C.