For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Polyphenol Oxidase - Manual
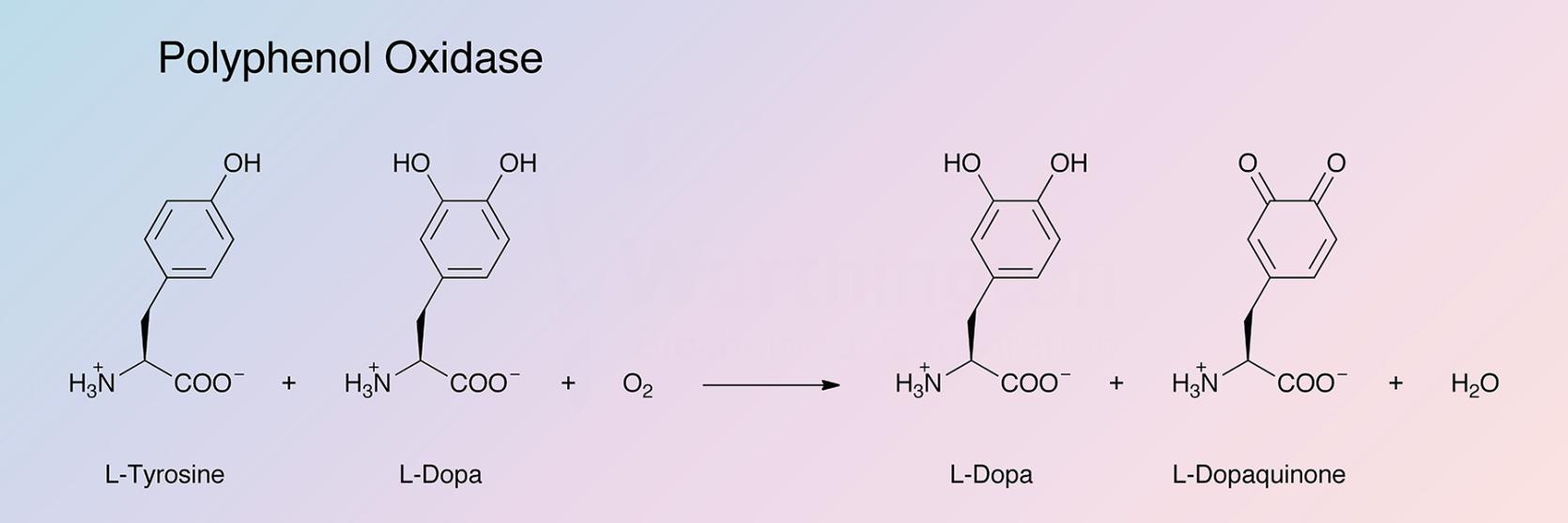
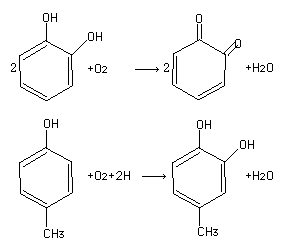
Polyphenol oxidase (tyrosinase) (TY) is a bifunctional, copper-containing oxidase having both catecholase and cresolase activity (Malmström and Rydén 1968):
Jolley et al. (1974) refer to it as an oxygen and 4 electron-transferring phenol oxidase. It is responsible for browning reactions throughout the phylogenetic scale.
Although a tyrosinase from Neurospora crassa has been purified (Fling et al. 1963), most work has been done with the mushroom enzyme, even though yields and consistency are poor; its multiplicity was shown by Smith and Krueger (1962). Bouchilloux et al. (1963) obtained four enzymes. See review by Nelson and Mason (1970).
Characteristics of Polyphenol Oxidase from Mushroom:
A large number of parasubstituted catechols areoxidized (Duckworth and Coleman 1970).
The enzyme is a tetramer containing four gram atoms of copper per molecule (Jolley et al. 1974), and two binding sites for aromatic compounds including phenolic substrates. There is also a distinctly different binding site for oxygen, the copper site (Duckworth and Coleman 1970). The copper is probably in the cuprous state; inactivation of the enzyme is associated with increase in Cu2+. (Kertész et al. 1972). The amino acid composition has been determined. Extensive structural studies have been reported by Jolley et al. (1969); and Duckworth and Coleman (1970). See also Jolley et al. (1972, 1973, and 1974).
128,000 (Duckworth and Coleman 1970).
6.0-7.0.
![]() = 24.9 (immediately after purification) (Duckworth and Coleman 1970).
= 24.9 (immediately after purification) (Duckworth and Coleman 1970).
Compounds that complex with copper. The enzyme is also inhibited competitively by benzoic acid with respect to catechol and by cyanide with respect to oxygen (Duckworth and Coleman 1970).
The lyophilized preparation is stable for 6-12 months when stored at -20°C.
Polyphenol oxidase is an oxygen transferring enzyme. Besides using O2 to catalyze the dehydrogenation of catechols to orthoquinones and the orthohydroxylation of phenols to catechols, a peroxidase activity has been reported on by Strothkamp and Mason (1974). Kinetic studies have been reported by Kertész et al. (1971). See also the review by Malmström and Rydén.