For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Lactate Dehydrogenase, L- (Cytochrome b2) - Manual
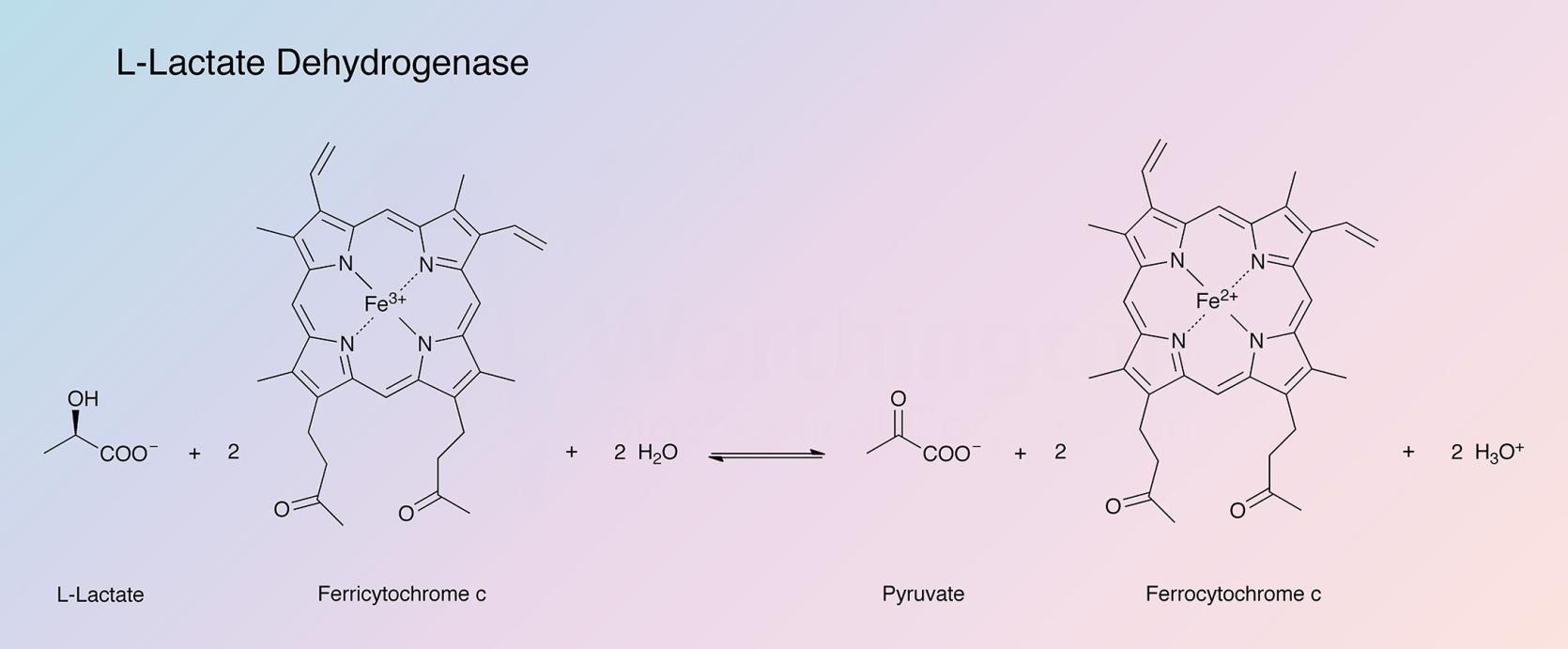
L-lactic acid dehydrogenase from baker's yeast (cytochrome b2), in nature, catalyzes the following reaction:
Other known oxidants which can be used in vitro are ferricyanide, phenozine methosulfate, certain redox dyes and quinone. A review has been written by Nygaard (1963). Preparation and properties have been summarized by Symons and Burgoyne (1966). An important use of this enzyme is in the specific, enzymatic determination of L-lactate. Schon (1965) describes a method utilizing Fe3+ phenanthroline complex. He indicates it to be 3 X more sensitive than that using NAD-dependent LDH from muscle. Another method is given below.
Characteristics of L-Lactate Dehydrogenase from Baker's Yeast:
The enzyme acts upon L-(+)-lactate, but not the D-isomer, and α-hydroxybutyrate. Electrons are transferred to ferricytochrome c, ferricyanide, methylene blue, 2,6-dichlorophenol indophenol and 1,2-naphthoquine-4-sulfate. Kinetic studies have been reported by Iwatsubo and Capeillere (1967).
Jacq and Lederer (1972 and 1974) and Guiard et al. (1973) indicate that the intact enzyme is tetrameric. Each of the identical subunits consists of a single polypeptide chain with one protoheme IX and one flavin mononucleotide as prosthetic groups. The latter have been reported on by Risler and Groudinsky (1973). Guiard et al. (1974) have determined the amino acid sequence in the heme binding region. The enzyme prepared by the crystallization method of Appleby and Morton (1959), without a protease inhibitor, shows a single cleavage of each of the four polypeptide chains; thus each monomer consists of an &@945;-chain of molecular weight 36,000 and a [[beta]]-chain, molecular weight 21,000 (Jacq and Lederer 1974).
228,000 (Jacq and Lederer 1974) (See also Guiard and Lederer 1975).
For cytochrome C: 5.5-9.0; for lactate and ferricyanide: 7.0-8.5.
![]() = 31.2 (Pajot and Groudinsky 1970).
= 31.2 (Pajot and Groudinsky 1970).
Heavy metals and oxygen (Armstrong et al. 1963) as well as glycerate, oxalate, malate, phenylpyruvate and fatty acids (Nygaard 1963).
The 0.65 saturated ammonium sulfate suspension is stable for at least six months at 2 - 8°C.
EDTA prevents metal inhibition.
Km=1.6 mM for lactate with ferricyanide.