For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Catalase - Manual
Catalase is an enzyme responsible for the degradation of hydrogen peroxide. It is a protective enzyme present in nearly all animal cells.
Bovine liver catalase was one of the first enzymes to be isolated to a high state of purity and the first iron-containing enzyme to be isolated (Sumner and Dounce 1937). The reaction mechanism was initially proposed to be a free radical mechanism by Oppenheimer and Stern in 1939. Throughout the next few decades, catalysis was determined to occur at the iron atom of the porphyrin (Warburg 1949, Keilin 1966, and Chance 1951). A more convenient method of preparing crystalline catalase from bovine liver was developed in 1952 by Tauber and Petit, and X-ray structure studies of the heme region of myoglobin examined the heme-containing active site (Stryer et al. 1964).
In the 1970s, X-ray and NMR studies provided further insight into the structure of the protein and the active site of hydroperoxidases in general (Larsson et al. 1970, and Hershberg and Chance 1975). In the 1980s, crystal structures of bovine liver catalases were published (Murthy et al. 1981, and Fita and Rossmann 1985).
In the 1990s, the inhibition of catalase by nitric oxide and the relation of this effect to nitric oxide cytotoxicity was investigated (Brown 1995). A study was conducted to compare the heme structures of catalases from various sources by Andersson et al. in 1995, and the unfolding and refolding of bovine liver catalase at various pHs and salt conditions was investigated by Prajapati et al. in 1998.
Recently, catalase has been investigated as a possible agent to support methods of intracellular drug delivery (Siwale et al. 2009). Catalase has also been incorporated into an assay for cholesterol quantification (Robinet et al. 2010) and a biosensor for alcohol determination (Hnaien et al. 2010).
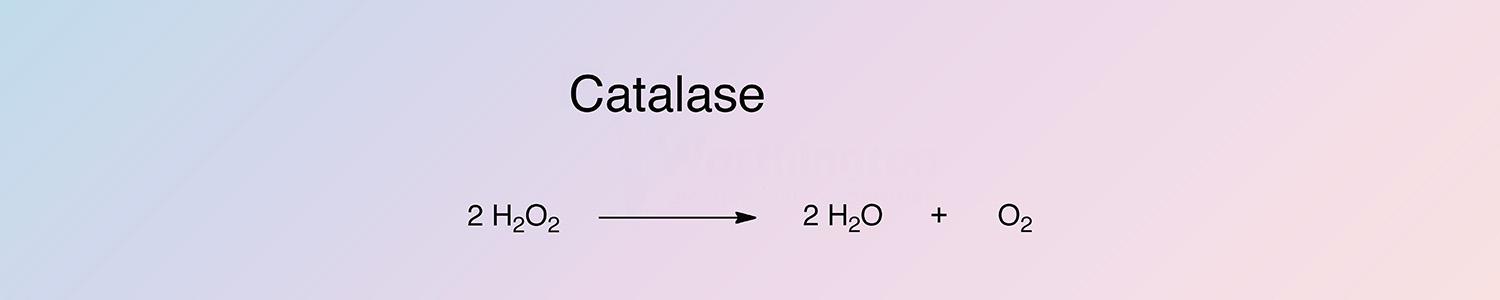
The reaction of catalase occurs in two steps. A molecule of hydrogen peroxide oxidizes the heme to an oxyferryl species. A porphyrin cation radical is generated when one oxidation equivalent is removed from iron and one from the poryphyrin ring. A second hydrogen peroxide molecule acts as a reducing agent to regenerate the resting state enzyme, producing a molecule of oxygen and water (Switala and Loewen 2002).
The gene that encodes catalase, CAT, is located on chromosome 15 in Bos taurus. The gene is conserved in human, chimpanzee, dog, mouse, rat, chicken, zebrafish, fruit fly, mosquito, C. elegans, S. pombe, S. cerevisiae, K. lactis, E. gossypii, N. crassa, A. thaliana, and rice (Gene ID: 531682).
Composition:
Catalase is a tetramer consisting of four identical, tetrahedrally arranged subunits. Each 60 kDa subunit contains a heme group and NADPH in its active center (Scibior and Czeczot 2006).
- Commercially wherever hydrogen peroxide is used as a germicide (Chu et al. 1975)
- Milk preservative with peroxidase (Collins 1971)
- Increases synthesis and stability of diacetyl in cultured milk (Collins 1971)
- Free radical research (Lardinois 1995)
- Deodorization (White and White 1997)
- Decomposition of residual hydrogen peroxide after bleaching woven and knitted cotton fabrics before drying (White and White 1997)
- Cysteamine determination (White and White 1997)
- Gluconic and glycolic acid production (Seip et al. 1994, and Godjevargova et al. 2004)
- Cleaning silicon and semiconductor plates (White and White 1997)
P00432
- 232 kDa (Schroeder et al. 1969)
- 240 kDa (Herskovits 1969)
- Monomer: 57.5 (Schroeder et al. 1969)
Approximately 7.0 (Maehly and Chance 1954)
- 5.4 (Samejima et al. 1962)
- 246,000 cm-1 M-1 (Theoretical)
- E1%, 276 = 12.9 (Herskovits 1969)
- Histidine (H74)
- Asparagine (N147)
- Sodium arsenate (Kertulis-Tartar et al. 2009)
- Cyanide, azide, hydroxylamine, aminotriazole, and mercaptoethanol (Switala and Loewen 2002)
- Ascorbate alone as well as with Cu2+ (Orr 1967a, b)
- Freezing and lyophilization cause inactivation (Tanford and Lovrien 1962, and Deisseroth and Dounce 1967).
- Inactivated by sunlight under aerobic conditions (Mitchell and Anderson 1965)
- Peroxide (Altomare et al. 1974)
- Nitro and nitroso compounds (Titov et al. 2008)