For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Introduction to Enzymes
Enzyme Kinetics: Energy Levels
Chemists have known for almost a century that for most chemical reactions to proceed, some form of energy is needed. They have termed this quantity of energy, "the energy of activation." It is the magnitude of the activation energy which determines just how fast the reaction will proceed. It is believed that enzymes lower the activation energy for the reaction they are catalyzing. Figure 3 illustrates this concept.
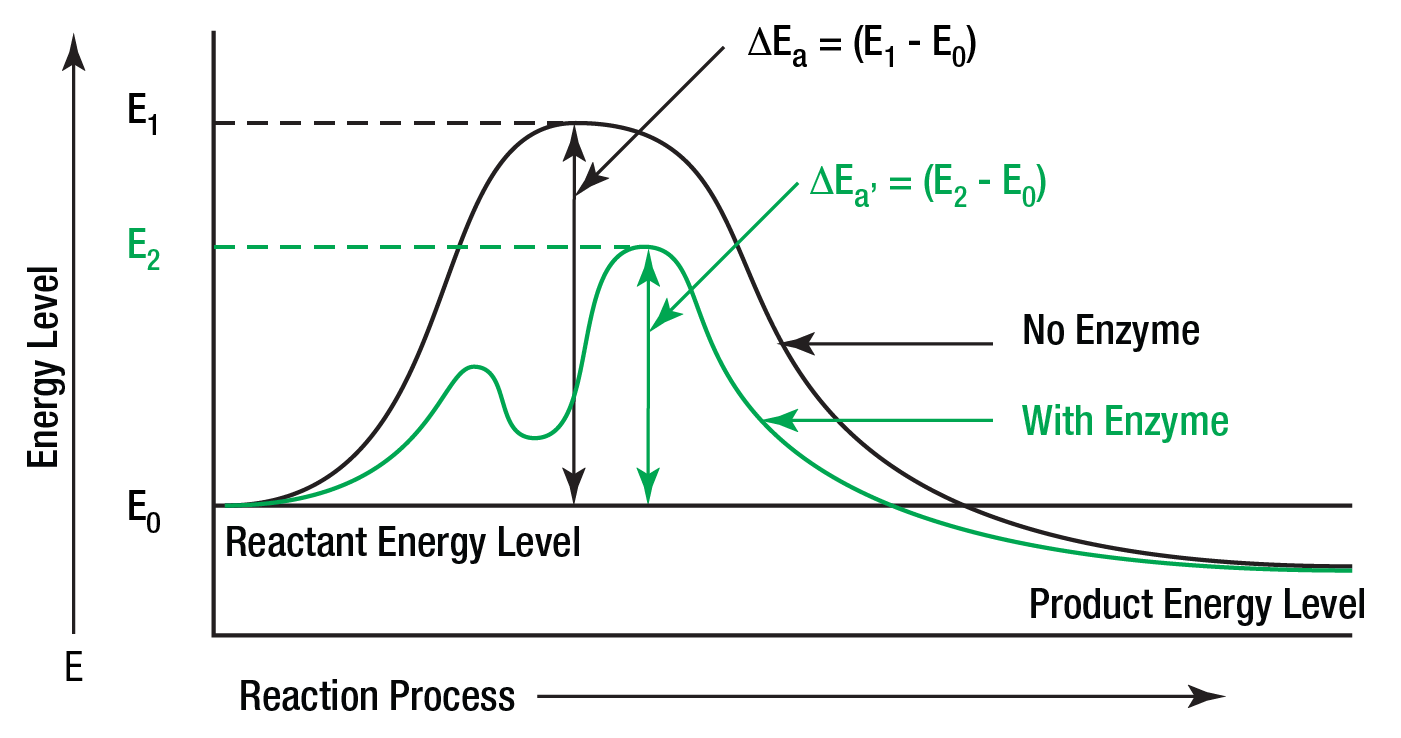
Figure 3: Energy of activation ΔEa is less than ΔEa’ due to the effect of the enzyme on the substrate.
The enzyme is thought to reduce the "path" of the reaction. This shortened path would require less energy for each molecule of substrate converted to product. Given a total amount of available energy, more molecules of substrate would be converted when the enzyme is present (the shortened "path") than when it is absent. Hence, the reaction is said to go faster in a given period of time.