For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Lysozyme - Manual
Lysozyme is an antimicrobial enzyme that is found in a wide variety of organisms including birds, mammals, plants, insects, and bacteria (Newman et al. 1974). The lysozyme of chicken egg white has been most extensively studied.
Lysozyme from chicken egg was first described by Laschtschenko in 1909 (Laschtschenko 1909). It was also reported in saliva by Bloomfield in 1919 (Imoto et al. 1972). Lysozyme was not officially named and understood to be present in many biological tissues and secretions until 1922 (Fleming 1922). During these experiments Alexander Fleming discovered Micrococcus lysodeikticus, a bacteria especially susceptible to lysozyme, which is still used today for lysozyme activity assays (Imoto et al. 1972).
In 1965, Blake et al. solved the structure of lysozyme, making it the second protein and first enzyme structure to be solved by X-ray diffraction methods (Blake et al. 1965). A year later, the mechanism was explained (Blake et al. 1966). Throughout the 1960s and into the 1970s, interest in the enzyme increased as a “natural” antibiotic and aid in the diagnosis of disease (Glynn 1968, Pruzanski and Saito 1969). Elevated lysozyme levels were found to be present in the urine and serum of leukemia patients (Osserman and Lawlor 1966 and Brierre et al. 1974), and in the cerebrospinal fluid of patients with a central nervous system tumor (Newman et al. 1974).
Lysozyme research in the 1980s included investigating enzyme intermediates (Acharya 1982, Desmadril and Yon 1984, and Ikegudri et al. 1986), analyzing the protein structure (Delepierre 1982), and performing binding studies (Nutta et al. 1988, Perraudin and Preels 1982, and Smitth-Gill et al. 1984). In the 1990s, transcription control, silencers, and additional binding sites were investigated (Bonifer et al. 1997, Baniahmad et al. 1991, and Madhusudan and Vijayan 1992).
Recent research has focused on obtaining more information about gene regulation of lysozyme both in the hen and other animals (Shimizu et al. 2005), gaining a better understanding of the secondary structure (Schwinté et al. 2002) and refining its use in biochemical applications (Reischl 2004 and Zhu 2006).
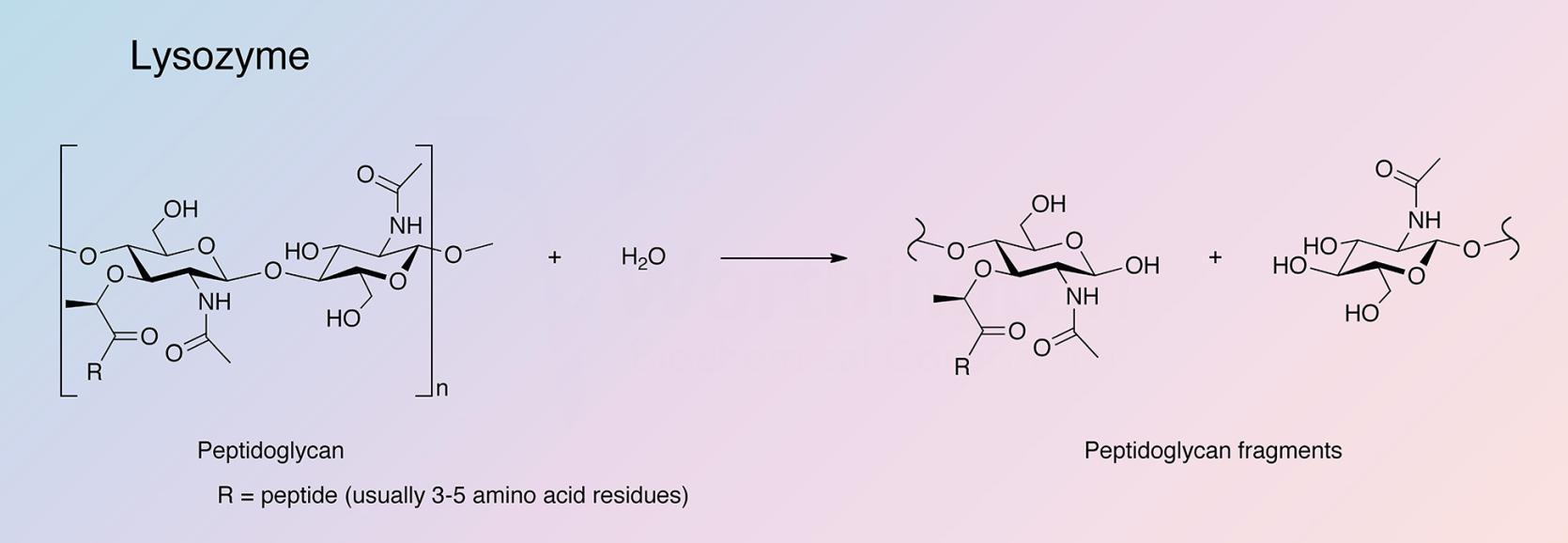
Lysozyme hydrolyzes the beta-glycosidic linkage between N-acetylmuramic acid and N-acetyl glucosamine in the peptidoglycan of bacterial cell walls and can also bind polymers of N-acetyl glucosamine (Arnheim et al. 1972).
The structure of lysozyme is consistent under a variety of conditions, making it ideal for crystallography studies. The active site of lysozyme consists of a deep crevice, which divides the protein into two domains linked by an alpha helix. One domain (residues 40 to 85) consists almost entirely of beta-sheet structure, whle the second domain (residues 89-99) is more helical (Strynadka and James 1991).
The mature lysozyme of chicken is composed of 128 amino acids. Amino acid sequences of other avian lysozymes are homologous in sequence and differ in only 4 to 20 amino acids (Arnheim et al. 1973). The lysozyme gene in chickens is expressed tissue specifically in the oviduct and in macrophages. Despite there only being one copy of the lysozyme gene, it is regulated differently in the oviduct and macrophages. Regulation of lysozyme in the oviduct utilizes steroid hormones, while a combination of cis-regulatory elements are used during differentiation in macrophages (Shimizu et al. 2005). Transcription is controlled by three enhancers, a complex promoter, and a negative regulatory element (Bonifer et al. 1997, and Lefevre et al. 2008).
- Nucleic acid preparation (Taylor and Utter 1974)
- Protein purification from inclusion bodies (Reischl 2004)
- Plasmid preparation (to break down membranes and cell wall) (Zhu 2006)
- Hydrolysis of chitin (Hayashi et al. 1969)
- Hydrolysis of bacterial cell walls (Shockman et al. 1996)
P00698
- Class: Mainly Alpha
- Architecture: Orthogonal Bundle
- Topology: Lysozyme
14.3 kDa (Theoretical)
6.0-9.0 (Davis et al. 1969)
9.32 (Theoretical)
- 38,940 cm-1 M-1 (Theoretical)
- E1%,280 = 27.21 (Theoretical)
- Glutamic acid (E53)
- Aspartic acid (D70)
- EDTA
(White and White 1997)
- SDS
- Alcohols
- N-acetyle-D-glucosamine
- Oxidizing agents
(White and White 1997)