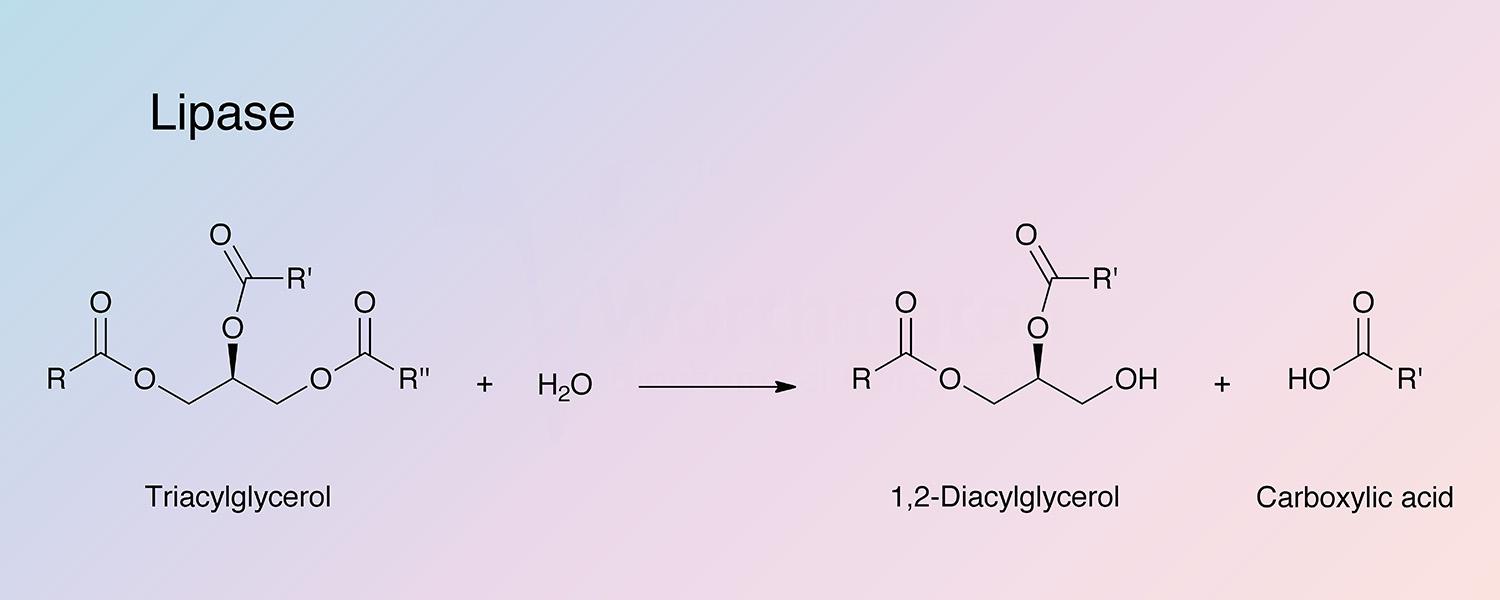
Lipase - Manual
Pancreatic lipase (PL),one of the exocrine enzymes of pancreatic juice, catalyzes the hydrolysis of emulsified esters of glycerol and long chain fatty acids. The substrate is not a single molecule but a nonaqueous phase of aggregated lipid (Brockerhoff and Jensen 1974). The operative substrate characteristic is aggregates of ester molecules, micelles or monomolecular film, interfacing an aqueous medium. Enzyme activity is directly related to the concentration of substrate molecules on the interface (Esposito et al 1973; Lagocki et al 1973. PL attacks the primary ester groups most readily. Monoglycerides are poor substrates (it is the 2-monoglycerides that are absorbed through the intestinal wall and reformed into lymph chlyomicrons). Pancreatic lipases have been thoughly reviewed by Brockerhoff and Jensen (1974), and Desnuell (1972). Liberman and Ollis (1975) have reported on lipase immobilized on stainless steel and polyacrylamide beads. Using a fluidized bed recycle reactor it is indicated that enzyme-substrate affinity is not altered.
Characteristics of Lipase from Porcine Pancreas:
Two lipases are present. Lipase A is more acidic than Lipase B; otherwise, the two isoenzymes are nearly the same (Verger et al 1969). Normally, a cofactor is bound to the enzymes (Maylie et al 1971). Two co-lipases were purified by Erlanson et al (1973). They were quite similar polypeptide chains with a molecular weight of 11,000. See also Borgstrom et al (1974). Borstrom and Earlanson (1973) indicated that co-lipase might be classified as a co-enzyme for lipase in that they interact in a stoichiometrical relationship.
PL has a broad spectrum of side chain specificity (Lagocki et al 1973). See also Savary (1972) and Brockerhoff (1969a)
The amino acid composition, which is almost identical except for isoleucine, is shown in Brockerhoff and Jensen (1974)-(Table IV-3, pg 43). Both contain a carbohydrate moiety (Garner and Smith 1972). Histidine is involved in the active site (Semeriva et al 1971). See Hultin (1992). Modification of the free carboxyl group by amide formation inactivates the enzyme (Semeriva et al 1972). According to Desnuelle (1972) the carboxyl in lipase stabilizes the active enzyme, i.e., the enzyme conformation resulting from adsorption at a hydrophobic interface. Although PL contains two disulfide groups, they are not involved in enzymatic activity (Verger et al 1971). Diisopropylphosphofluoridate (DFP) binds to a tyrosine residue but it is not inhibitory (Maylie et al 1969). See also Rovery et al. (1973)
45,000-50,000 (Verger et al 1969)
Lipase A= 4.9 (Brockerhoff and Jensen 1974) and Lipase B=5.0
![]() =13.3 (Desnuelle 1972)
=13.3 (Desnuelle 1972)
Ca2+ is required for activity [Sr2+ and Mg2+ are less effective activators (Sarda et al 1957)].
Versene, Zn2+ ,Cu 2+, Hg2+,iodine, PCMB (Willis 1960). DFP does not inhibit.
Highly purified, homogenous preparations of hog pancreas lipase are extremely labile.
See Desnuelle (1972) on "Catalytic Properties" (page 586). Momsen and Brockman (1976a and b) report the effects of taurodeoxycholate and co-lipase. At low concentrations, up to 0.3mM, the bile salt increases the stability of the lipase to 5 fold. At higher levels (0.3-0.8mM), but below the critical micelle concentration, it interferes with enzyme adsorption on the substrate interface, thus inhibiting lipolysis. Co-lipase counters this inhibitory effect by providing high affinity binding sites at the surface of the lipase-bile salt complex. See also Borgstrom and Elanson (1973), Borgstrom et al (1974), and Kaimal and Saroja (1989). Co-lipase without bile-salts only mildly stimulates activity. Brockman et al (1973) report on PL activity toward soluble triglycerides such as tripropionin. It is stimulated in the presence of hydrophobic surfaces. Santhanam and Wagle (1971) indicate that protein kinase, Mg2+ , ATP and cAMP stimulate PL activity.
DFP may be used to stabilize impure preparations containing proteinases in solutions.