Nitrate Reductase - Manual
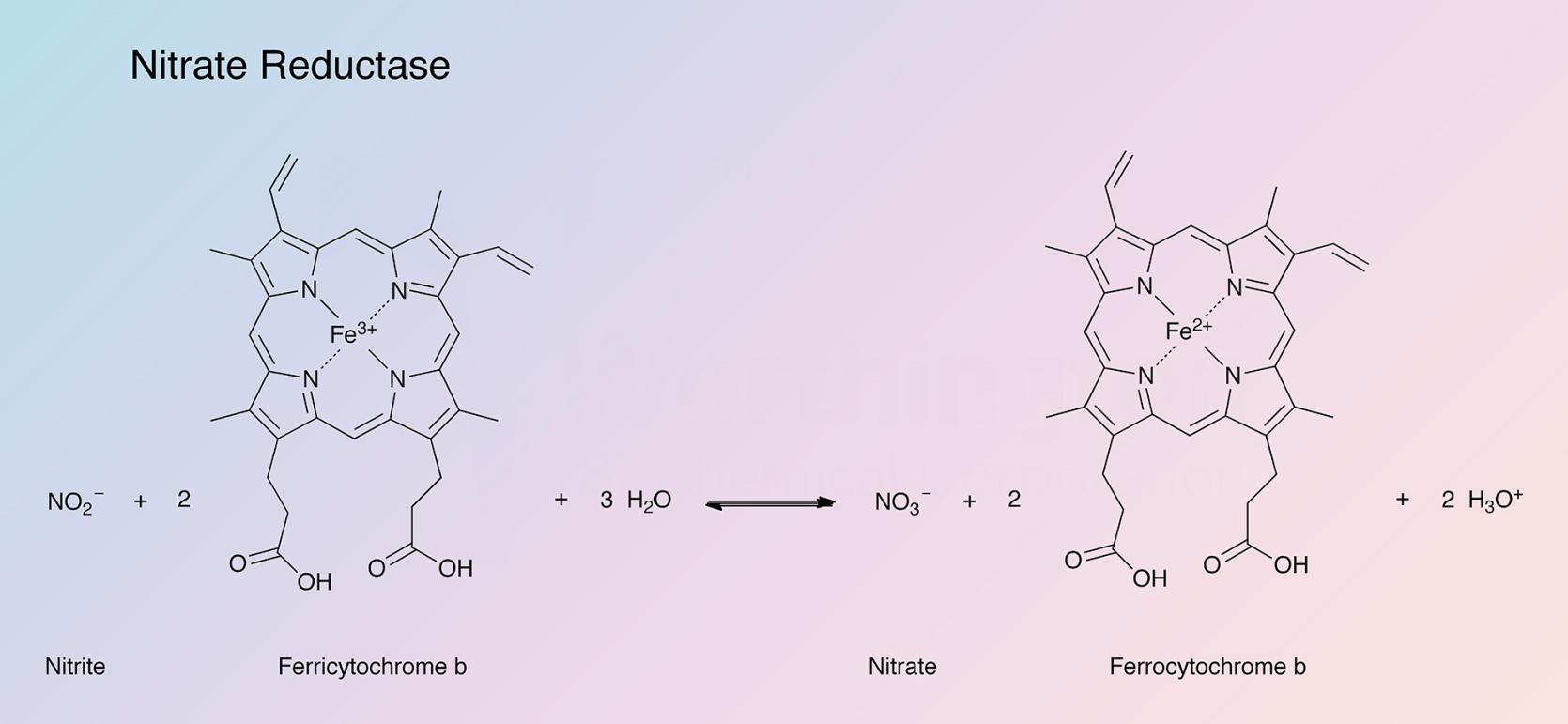
This respiratory or "anaerobic" nitrate reductase (NR) (Cytochrome) from E. coli catalyzes the following reaction:
It is a membrane-bound enzyme closely associated with formate dehydrogenase which, along with NR contains a functional cytochrome b. An electron transport scheme from formate to nitrate and including possible quinone participation has been reported by Enoch and Lester (1974). Despite disruption of the system and removal of cofactors, purified NR remains functional in the formation of nitrite when a suitable reducing agent is supplied. In such cases reduced methyl viologen and benzyl viologen have been found most useful.
The use of viologen dyes which may be reduced chemically serves both in the assay of the enzyme and in the determination of low concentrations of nitrate. Lowe and Gillespie (1975) describe a very sensitive colorimetric nitrate determination. (The nitrite formed reacts with a chromogen reagent to yield a red color.) The test is adaptable for ground and stream water pollution studies. See also McNamara et al. (1971).
Characteristics of Nitrate Reductase from E. coli:
Both nitrate and chlorate are substrates. The maximum specific activity reported was 0.27 mM/minute by Der Vartanian and Forget (1975). Reduced benzyl and methyl viologen, FMNH2 and FADH2 may be electron donors but NADH and NADPH are not suitable. (Forget 1974).
NR contains both iron and molybdenum. MacGregor et al. (1974) indicate there to be four atoms of Mo and four active sites per enzyme molecule. Although earlier investigators have claimed that the enzyme contains neither flavin nor heme, Enoch and Lester (1974) have shown heme to be a part of the molecule. A point generally agreed on is that the iron is closely associated with protein sulfur at nearly a 1:1 ratio.
Originally determined by Taniguchi and Itagaki (1960) as 1,000,000, the molecular weight was most recently determined by Enoch and Lester (1975) to be 498,000 in a Triton X100 solution. The latter authors describe the structure as a complex of three polypeptides of molecular weights 155,000, 63,000, and 19,000 (237,000 total, assuming one each) which suggests that they occur together in a dimer to make up most of the 498,000, and perhaps a tetramer to give the one million weight originally described.
Comparison with the results of Van't Riet and Planta (1975) who studied the nitrate reductase of Klebsiella aerogenes (a bacterium very similar to E. coli) may be helpful. They found the Klebsiella aerogenes enzyme to be a one million dalton tetramer of which each monomer was composed of four units: 117,000, 57,000, and two 52,000 molecular weight parts. MacGregor et al. (1974) also examined the E. coli enzyme and concluded that the molecule is composed of four 142,000 molecular weight polypeptides plus four of 58,000 molecular weight totaling 800,000 daltons.
In an attempt to clarify the confusion surrounding the molecular weight of E. coli nitrate reductase, Clegg (1975) has reexamined the enzyme and concluded that several different forms exist with differing subunit compositions. The suggestion by MacGregor et al. (1974) of a protease assisting the release of the enzyme from the membrane may account for some of the disagreement concerning size and make-up.
7.1.
4.2 (Forget 1974).
Cyanide and azide are strong inhibitors. Forget (1974) indicates the cyanide inhibition is competitive, but that the affinity of cyanide to the enzyme is about 1,000 times that of nitrate. Chloromercuribenzoate and iodoacetate are not inhibitory at about 1 mM concentrations (Forget 1974). Chelating agents, o-phenanthroline, 8-hydroxyquinoline, etc., have mild inhibitor effect; EDTA has none. (Taniguchi and Itagaki 1960).
In purified form, the stability is moderate. Forget (1974) recommends storage at 0-1deg.C as a precipitate in 60% saturated ammonium sulfate.