For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Cholesterol Esterase - Manual
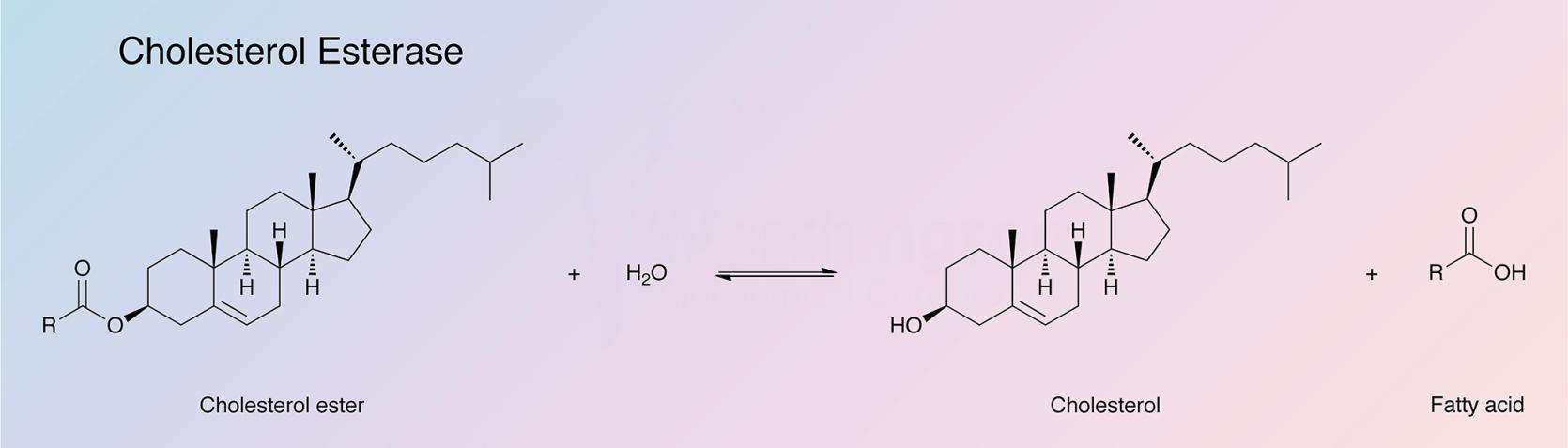
Cholesterol esterase catalyzes the hydrolysis of sterol esters into their component sterols and fatty acids. The enzyme is found primarily in the pancreas, but has been detected in other tissues as well. Bile salts, such as cholate and its conjugates, are required to stabilize the enzyme in its native polymeric form and to protect it from proteolytic hydrolysis in the intestine (Vahouny and Treadwell 1968). Cholesterol esterase finds clinical applications in the determination of serum cholesterol (Allain et al. 1974).
Throughout the early 1900s the enzymatically catalyzed synthesis and hydrolysis of cholesterol esters in the presence of certain tissues was observed and described (Kondo 1910, Schultz 1912, Cytronberg 1912, Gardner and Lander 1913, Mueller 1916, Porter 1916, Nomura 1924, Shope 1928, Nedswedski 1935, Klein 1939, Sperry 1935, and Sperry and Stoyanoff 1937).
In 1949 and 1950, two papers were published as part of an extensive study on cholesterol esterase. This research described a method for crude enzyme preparation that focused on the importance of stable emulsions of cholesterol and cholesterol esters for activity (Yamamoto et al. 1949, and Swell and Treadwell 1950). Throughout the 1950s, the cholesterol esterase of serum and various other tissues was studied (Korzenovsky 1960). In 1957, Hernandez and Chaikoff studied the properties of pancreatic cholesterol esterase specifically and devised a rapid turbidimetric method for enzymatic activity estimation (Hernandez and Chaikoff 1957).
In the 1960s, Vahouny et al. studied methods for protecting cholesterol esterase from proteolytic inactivation (Vahouny et al. 1964). This study coupled with the known correlation between cardiovascular disease and high serum cholesterol (Keys 1980, and Goldstein and Brown 2003) led to the development of total serum cholesterol determination methods using cholesterol esterase (Allain et al. 1974).
In 1989, Kyger et al. sequenced a cDNA clone of bovine pancreatic cholesterol esterase. In 1994, the landmark “4S” study showed for the first time that lowering LDL levels through the use of statins could prevent heart attacks and prolong life (Goldstein and Brown 2003). With such treatment options available and the need for testing total cholesterol in serum rising dramatically, cholesterol esterase has become one of the most widely used enzymes in clinical laboratories (MacLachlan 2000).
Cholesterol esterase is synthesized in the acinar cells of the pancreas, stored in zymogen granules, and secreted as a component of pancreatic juice into the lumen of the small intestine (Labow et al. 1983). Cholesterol esterase hydrolyzes a wide range of ester substrates including cholesteryl esters, acylglycerides, phospholipids (Brockerhoff and Jensen 1974), retinyl esters (Fredrikzon et al. 1978), vitamin esters, and phenyl esters (Rudd and Brockman 1984). The enzyme has also been found to have amidase activity (Hui et al. 1993). The enzyme is useful as a biocatalyst because of its ability to catalyze transacylation reactions in a water-limited environment (Gallo et al. 1977, Kazlauskas 1989, and Feaster et al. 1996).
Cholesterol esterase is a glycoprotein that in the presence of salts aggregates to a hexamer (Hyun et al. 1971). Cholesterol esterase belongs to the alpha/beta-hydrolase fold family (Ollis et al. 1992, and Cygler et al. 1993). Most members of this family are esterases and share secondary and tertiary features. Nearly all use a serine esterase catalytic mechanism, which resembles that of the serine proteases (Kraut 1977).
The gene that encodes cholesterol esterase in pigs (lipA) is located on chromosome 14 (GENE ID: 100142668). The LIPA gene is conserved in human, chimpanzee, dog, cow, mouse, rat, chicken, zebrafish, fruit fly, mosquito, C. elegans, S. pombe, S. cerevisiae, E. gossypii, M. grisea, N. cassa, A. thaliana, and rice.
- Determination of cholesterol in serum and plasma, with cholesterol oxidase or peroxidase
- Synthesis of optically active alcohols and carboxylic acids (via ester hydrolysis, esterification, or transesterification)
NP_001116606
- Monomer: 65 kDa (Hyun et al. 1971)
- Monomer: 43.3 kDa (Theoretical)
- Hexamer: 400 kDa (Hyun et al. 1971)
- For esterification, 6.1-6.2 (Vahouny and Treadwell 1968)
- For hydrolysis, 6.6-7.0 (Vahouny and Treadwell 1968)
- 7.91 (Theoretical)
- 86,680 cm-1M-1 (Theoretical)
- E1%,280 = 20.01 (Theoretical)
- Serine (S194)
- Histidine (H435)
- Aspartate (D320)
- Bile salts
- Cholate (Vahouny et al. 1964)
- Glycocholate (Vahouny et al. 1964)
- Taurocholate (Vahouny et al. 1964)
- PMSF and p-chloromercuribenzoate (Hyun et al. 1971, and Vahouny et al. 1964)
- Diisopropyl fluorophosphate (Momsen and Brockman 1976)
- Hg2+, Ag+, and ionic detergents
- Aryl carbamates (Feaster et al. 1996)