For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Glycerol Kinase - Manual
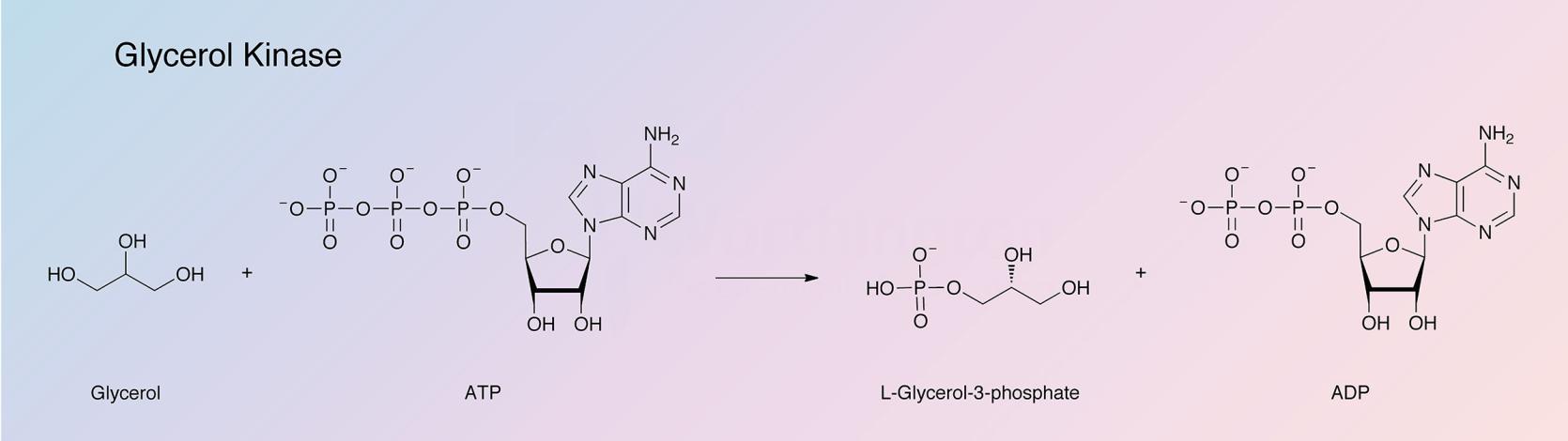
Glycerol kinase (GK) catalyzes the following reaction:
Glycerol kinase has been reviewed by Thorner and Paulus (1973a). The activity is found widely in nature. In microorganisms GK makes possible the utilization of glycerol as a carbon source. According to Kida et al. (1973), in mammals the enzyme represents a juncture of sugar and fat metabolism; however, the mammalian enzymes are quite different from the microbial. Yeast GK from Candida mycorderma has been reported on by and Cleland (1974) and Eisenthal et al. (1974).
The enzyme is important to the clinical chemist in the determination of glycerol. Pinter et al. (1967) also indicate that GK is useful in the assay of glyceraldehyde and dihydroxyacetone following their quantitative reduction to glycerol with sodium borohydride. Mallon and Dalton (1971) report on an automated glycerol assay.
Characteristics of Glycerol Kinase from E. coli:
Of all nucleotides tested, only ATP can act as phosphate donor. Dihydroxyacetone and glyceraldehyde are phosphorylated by a highly purified preparation but at greatly reduced rates (See Thorner and Paulus review, 1973).
Thorner and Paulus (1973b) indicate the enzyme to be composed of four identical subunits. They also report the purification of GK from a mutant strain with double the specific activity but of identical size and subunit components. (See also Berman-Kurtz et al. 1971). The amino acid composition has been reported (Thorner and Paulus 1971).
217,000 (Thorner and Paulus 1971).
9.0 - 9.8. Variations in the assay procedure may shift the optimum pH somewhat. (Hayashi and Lin 1967)
GK is protected by the presence of EDTA and mercaptoethanol and especially by the presence of glycerol. Maximum stability is at pH 7.0; it is relatively poor below pH 6.0 and above pH 7.6. The enzyme is insoluble in pure water and in buffers of low ionic-strength. Excellent stability has been reported for the crystalline enzyme in half-saturated ammonium sulfate at 0°C.
GK phosphorylates glycerol exlusively to L-α-glycerophosphate. The reaction is essentially irreversible and Mg2+ is required. Although manganous ions may be substituted the activity is reduced to about one third. (Hayashi and Lin 1967). The catalytic rate is slowed by fructose biphosphate (Zwaig and Lin 1966). Thorner and Paulus (1973) report on the allosteric properties of the enzyme.