For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Arginase - Manual
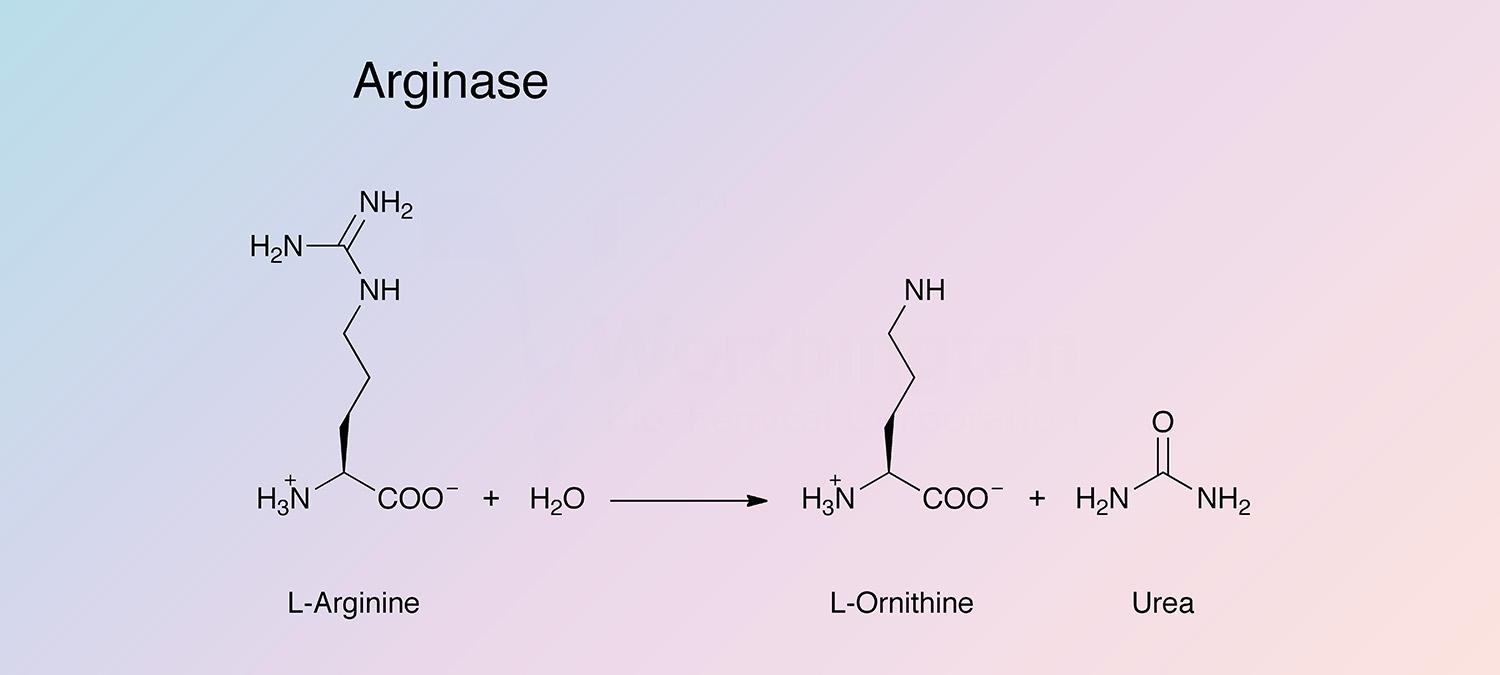
Arginase is a hydrolase present in many tissues and organs that catalyzes the following reaction:
The enzyme participates in the Krebs-Henseleit urea cycle. It is most highly concentrated in mammalian liver, and is also present in abundance in mammary glands, where the urea cycle is not present. There are two distinct types of arginase: Type I (liver-type) and Type II (mitchondrial).
In 1928, Krebs and Henseleit conducted a series of experiments using liver slices and manometric assays to show that in the presence of arginase, ornithine produced urea (Jenkinson et al. 1996). Crude preparations of arginase were reported as early as 1931 (Salaskin and Solowjew 1931, and Waldschmidt-Leitz et al. 1931). Hellerman and Perkins first showed activation by bivalent metal ions including cobalt, nickel, manganese, and iron (Hellerman and Perkins 1935). In 1940 an improved purification developed by Richards and Hellerman showed that the activity could be restored to pH-inactivated arginase by Mn2+ and (to a lesser extent) Fe2+ (Richards and Hellerman 1940).
In 1956, the “partial” purification was further improved by Robbins and Shields. They demonstrated that the activity of arginase was dependent upon manganese, and found the optimal pH to be 9.2 (which has since been adjusted to 9.4) (Robbins and Shields 1956, and Xie et al. 2004).
Much work was done investigating inhibitors in the late 1970s and early 1980s (Rosenfeld et al. 1975, Bedino 1977, and Pace and Landers 1981). Bedino studied the effect of the product/inhibitor ornithine, and proposed an allosteric model for the regulation of the enzyme’s activity (Bedino 1977).
Recent research has investigated the roles of arginases in vascular disease, pulmonary disease, infectious disease, and cancer (Zimmerman and Rothenberg 2006, Maarsingh et al. 2008, Santhanam et al. 2008, and Morris 2009). Varying levels of arginase have been found in the reproductive system of cattle (Razmi et al. 2005), as well as in the immune system of mice and humans (Munder 2009).
Arginase is highly specific for its substrate. Class I and Class II enzymes contain carboxy-terminal tyrosine residues required for maximal catalytic activity. Arginase has the highest level of specific activity of the urea cycle enzymes in the liver (Jenkinson et al. 1996).
Arginase is a homotrimer. Each subunit contains an active site, and the two essential manganese ions are bridged by oxygens and separated by approximately 3.3 Å. The mechanism is proposed to be a nucleophilic attack by the metal bridging hydroxide at the guanidinium carbon of the arginine substrate (Ash 2004).
Arginase I and arginase II are each encoded by a different gene. Arginase I is located in the cytoplasm and expressed in the liver as part of the urea cycle. Arginase II is a mitochondrial enzyme, and is expressed primarily in the kidney (Romero et al. 2008). Based on sequence analysis, arginase is probably a primordial enzyme that was present in the universal common ancestor (Ouzounis and Krypides 1994). The arg1 gene is conserved in several species including human, dog, mouse, rat, zebrafish, and S. cerevisiae. The gene is induced in the late fetal period and is upregulated by high nutritional protein uptake (Morris et al. 1989). This is mediated by glucocorticoids and glucagon (Morris et al. 1987).
A cis element is present around -90 to -51 relative to the start site and is overlapped by two protein binding regions. These regions have been identified as footprint areas A and B. A is more upstream and binds a CCAAT/enhancer binding protein (C/EBP) related factor, while B is recognized by two other mutually exclusive factors (Takiguchi and Mori 1991, and Chowdhury et al. 1996). An enhancer region is also present 11 kb downstream from the start site (Gotoh et al. 1994). Hepatocyte nuclear factor-4 (HNF-4) has been shown to repress the C/EBP stimulated arginase promoter activity (Chowdhury et al. 1996).
- Preparation of L-Ornithine
- Determination of L-Arginine in plasma and urine
Q2KJ64
- Class: Alpha Beta
- Architecture: 3-Layer(aba) Sandwich
- Topology: Arginase; Chain A
105.0 kDa (Theoretical)
9.4 (Xie et al. 2004)
- 5.9 (Harell and Sokolovsky 1972)
- 74,340 cm-1 M-1 (Theoretical)
- E1%,280 = 7.08 (Theoretical)
- Histidine (H101, H126)
- Aspartate (D124, D128, D232, D234)
(Residues coordinate bound manganese)
- Mn2+, Mg2+, Ca2+, Ni2+, and Co2+ (Dabir et al. 2005)
- Indyl amino acid derivatives (Hrabak et al. 2008)
- EDTA (Esch et al. 1998)
- L-Proline and branched-chain amino acids (Dabir et al. 2006)
- Adenosine, inosine, uric acid (Rosenfeld et al. 1975)
- Possibly adenine (Rosenfeld et al. 1975, disputed by Pace and Landers 1981)