For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Carbonic Anhydrase - Manual
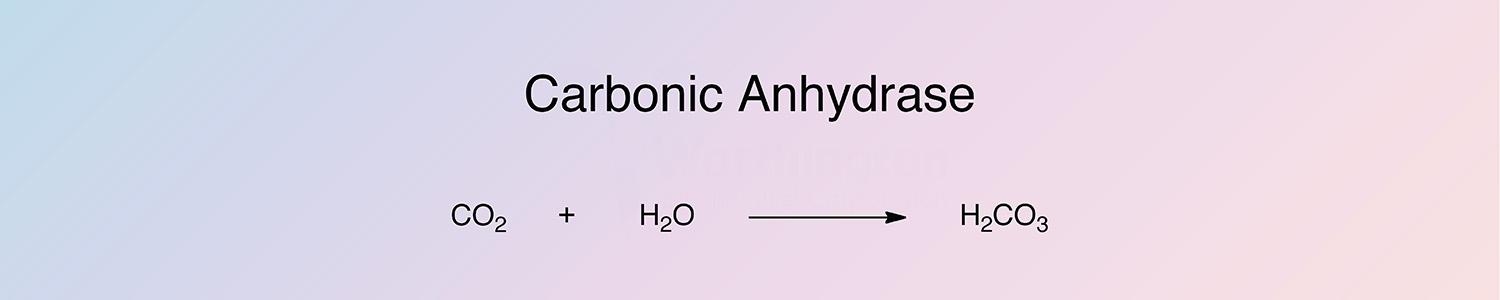
Carbonic anhydrase (CA) catalyzes the reversible hydration and dehydration reactions of CO2/H2CO3. CAs are widespread in nature, being found in animals, plants, and certain bacteria. Sixteen isozymes have been identified and characterized in mammals. Since erythrocyte CA is relatively easy to obtain for experimental purposes, it is the most widely studied.
CA was first discovered in vertebrate erythrocytes (Brinkman et al. 1932). It was inferred that a catalyst was present in blood that could dehydrate bicarbonate and allow it to escape as CO2 (Stadie and O’Brien 1933). CA was soon isolated from erythrocytes by Meldrum and Roughton in 1933. Upon the discovery in 1940 that zinc is an intrinsic cofactor, CA became the first recognized metalloenzyme (Keilin and Mann 1940). It was also deduced that sulfanilamide is an inhibitor of the enzyme, which led to pharmacological investigations and the eventual important discovery of CA inhibitors as treatment for glaucoma (Mann and Keilin 1940, Maren 1995, and Khalifah 2003).
It took over twenty years for CA to gain the interest of the research community, and it did so because of the simple substrates upon which it acts. It became known as one of the most efficient acid-base catalysts (Eigen and Hammes 1963). Interest in the enzyme was also gained when Lindskog replaced the zinc ion with cobalt and found the activity of the enzyme was unaffected (Lindskog and Malström 1962). This led to many studies on the oxidation of the metals and theories that the activity of the enzyme is related to ionization of nearby groups (Whitney et al. 1967, Appleton and Sarkar 1974, and Shinar and Navon 1974).
Human erythrocytes were used in studies of the 1970s to isolate and obtain the first amino acid sequences of CA-I (Andersson et al. 1972, and Lin and Deutsch 1973) and CA-II (Henderson et al. 1973, and Lin and Deutsch 1974). John Edsall performed extensive studies on the kinetics of human erythrocyte CA, and also discovered the first competitive inhibitor, imidazole (Edsall 1968, and Khalifah 2003).
Current research on CA continues to investigate the mechanism of inhibitor binding (Safarian et al. 2007) and CA’s role in inhibiting ectopic cardiovascular calcification (Rajachar et al. 2009).
Blood CO2 transport and excretion is largely dependent on the rapid catalysis of the CO2 reactions within the erythrocyte by CA (Tufts et al. 2003). Bovine CA reversibly hydrates alkyl pyruvates and it exhibits hydratase activity toward a wide variety of substrates (Pocker et al. 1974, and Wells et al. 1975).
Sixteen CA isozymes have been described so far in mammals. Erythrocyte CAs, CA-I and CA-II, are most well known. CA-I, CA-II, CA-III, CA-VII, and CA-XIII are cytosolic. CA-IV, CA-IX, CA-XII, CA-XIV, and CA-XV are membrane bound. CA-VI is secreted in saliva. CA-VA and CA-VB are mitochondrial. There are also three acatalytic forms referred to as CA-related proteins (CARPs): CARP-VIII, CARP-X, and CARP-XI (Coban et al. 2009).
The zinc metal is always bound to histidines 93, 95, and 118 (mature chain numbering). A hydrogen bonded network, linked to the zinc-bonded water molecule and these histidines either directly or indirectly, includes 28-Ser, 91-Glu, 105-Glu, 106-His, 116-His, 193-Tyr, 198-Thr, 208-Trp, and 223-Asn. These residues have been found to be highly conserved (Lindskog 1982, and Lindskog et al. 1984). Bovine and human CA I and II contain a unique C-terminal knot structure, which has been shown to be important in enzymatic and mechanical properties (Alam et al. 2002).
Each isozyme from erythrocytes (CA-I and CA-II) is composed of a single chain peptide of 259 or 260 amino acid residues. The low activity form (CA-I) contains 260 residues, while the high activity form (CA-II) contains 259 residues. Erythrocyte high and low activity forms within a given species usually show greater than 50% sequence identity; for example, equine CA-I and CA-II forms only show 55% identity (Wendorff et al.1985). In contrast, the same forms from different species show much greater homology; human CA-II and bovine CA-II show 77% sequence homology (Tashian et al. 1980, Engberg et al. 1985, Alam et al. 2003). The isozymes are encoded by separate genes but given the great deal of homology, especially in the active center, they appear to have a common evolutionary history. Ubiquitin, a 76 residue protein with some enzymatic properties of CAs, has a distinct sequence homology to CA (Deutsch 1987).
- CO2 determination in blood
- Elimination of CO2 in reagents for acidity testing
- Carboxy group transfers
- Reduction reactions
P00921
- Class: Alpha Beta
- Topology: Carbonic Anhydrase II
- 29.0 kDa (Theoretical)
- 30 kDa (Lindskog et al. 1971)
7.0-7.5 (Demir et al. 2000, and Tasgin et al. 2009)
6.40 (Theoretical)
- 50,070 cm-1M-1 (Theoretical)
- E1%, 280 = 19.0 (Nyman and Lindskog 1964)
- Histidine (H63)
- Asparagine (N66)
- Lysine (K126)
- HPO42- (Rowlett et al. 1991)
- SO32- (Rowlett et al. 1991)
- Monovalent anions (Lindskog et al. 1971, and Ward and Cull 1972)
- Sulfonates and sulfonamides (Pocker and Watamori 1973, and Binford et al. 1974)
- Imidazole (Edsall 1968)