For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Carboxypeptidase A - Manual
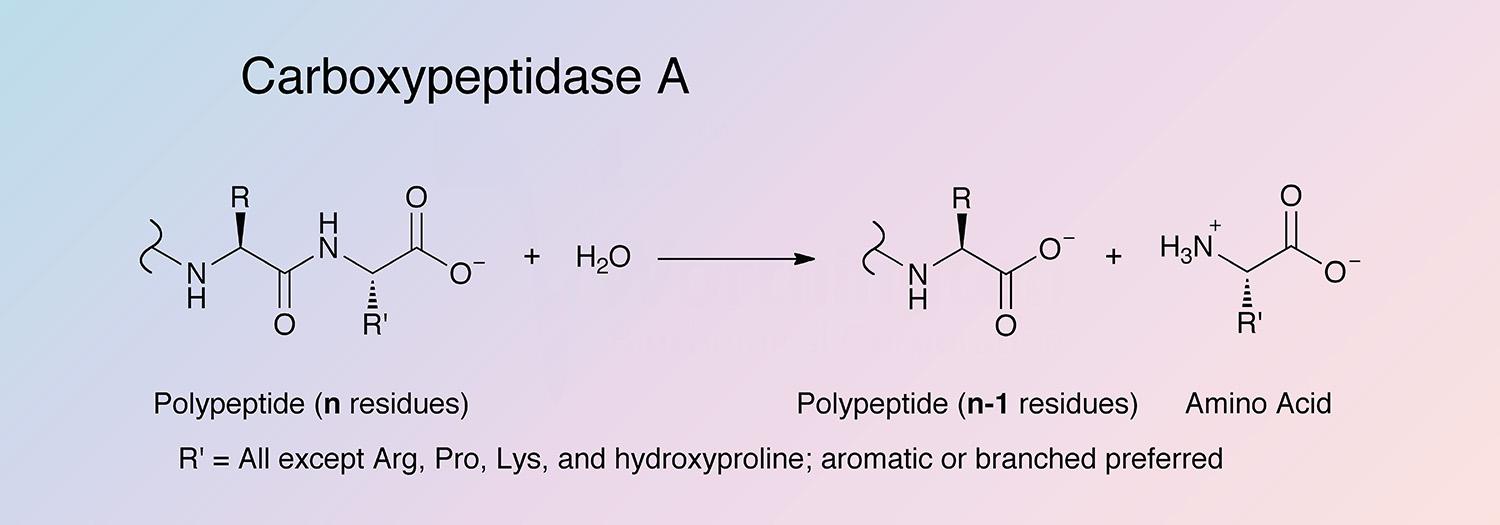
Carboxypeptidase A (CPDA) is a pancreatic metalloexopeptidase that hydrolyzes the peptide bond adjacent to the C-terminal end of a polypeptide chain.
CPDA was first isolated by Waldschmidt-Leitz and Purr in 1929 and first crystallized by Anson in 1937 (Hartsuck and Lipscomb 1971, and Auld 2004). CPDA was the first metalloprotease and second zinc enzyme to be identified (Vallee and Neurath 1954, and Auld 2004).
In 1953 Thompson preliminarily determined the N-terminal amino acid sequence, establishing that CPDA was a single polypeptide chain. Through x-ray diffraction studies Reeke et al. resolved the structure of CPDA to 2.0 Å resolution and determined CPDA is composed of 307 amino acid residues (Reeke et al. 1967). Folk and Schirmer isolated porcine CPDA, and Folk established the relationships between the two forms of the enzyme (Folk and Schirmer 1963, and Folk 1963).
CPDA was one of the first enzymes to have its complex kinetic profile extensively studied (Slobin and Carpenter 1966, Whitaker 1966, Auld and Vallee 1970, Auld and Vallee 1971, Hass and Neurath 1971, Naik and Horton 1973, and Canonici and Behnke 1974). In 1969, Bradshaw et al. determined the amino acid sequence of bovine CPDA.
The mechanism of activation of CPDA was used as a model to investigate the role of zymogens in biological systems (Neurath 1984), and in the 1990s the gene of bovine pancreatic proCPDA was identified (Le Huërou et al. 1991, and Goo et al. 1995).
Recent studies have investigated binding of inhibitors through kinetic studies (Chong and Auld 2007) and the generation of an enzyme-inhibitor crystal structure (Arolas 2005). CPDA has also been used for the development of C-terminal affinity tags (Austin et al. 2010).
C-terminal L-amino acids with aromatic or branched sidechains are preferentially cleaved (Libscomb 1970), and ester bonds of peptides with a free C-terminal carboxyl group can also cleaved by CPDA. Acylated -amino and -hydroxy carboxylic acids are also substrates (Auld 2004).
CPDA is secreted as a proenzyme, with a 94 amino acid segment that is cleaved by trypsin during activation. The active enzyme is composed of 309 amino acid residues (Guasch et al. 1992). Only one form of CPDA has been found in cattle, while two forms have been found to exist in humans and rats. The monomeric form of the proenzyme is found in most species; however, it also can be found as a binary or ternary complex. When it occurs as a binary complex, it is complexed with either chymotrypsinogen C or proproteinase E, and when it occurs as a ternary complex it is complexed with both (Gomis-Rüth et al. 1995, and Vendrell et al. 2000). The activation segment lies at the center of the three subunits.
Arg145 is believed to be the site that interacts with the substrate’s free alpha-carboxyl group, and Glu270 is the principle catalytic moiety (Hartsuck and Lipscomb 1971, and Rees et al. 1983).
When Bradshaw et al. determined the complete amino acid sequence they confirmed that bovine CPDA exists in two allotypic forms (Bradshaw 1969b, and Goo et al. 1995). One form of the enzyme contains Ile179, Ala228, and Val305, while the other contains Val179, Glu228, and Leu305 (Petra et al. 1969). In 1995, Goo et al. isolated from bovine pancreas the major CPDA that contains Ile179, Ala228, and Val305. The nucleotide sequence showed an open reading frame encoding a 16 amino acid signal peptide, a 94 amino acid activation peptide, and a 309 amino acid mature CPDA (Goo et al. 1995).
The amino acid sequences of the family to which CPDA belongs to (M14) are between 60 and 80% conserved. Conserved residues believed to be important in specificity and catalysis include Glu270, Arg145, Arg127, Tyr248, and Tyr198 (Auld 2004). In 2009 Zimin et al. assembled the entire Bos taurus genome.
- Resolution of racemic amino acids
- Sequence analysis
- Hydrolysis/condensation of amide bonds
- Downstream processing of fusion proteins that may precipitate at high salt concentrationes
- (White and White 1997)
P00730
- Class: Alpha Beta
- Architecture: 3-Layer(aba) Sandwich
- Topology: Aminopeptidase
- 35.3 (Bradshaw et al. 1969b)
- 34.8 (Theoretical)
7-9, depending on the substrate
6.08 (Theoretical)
- 65,670 cm-1M-1
- E1%, 278 = 19.4
- Arginine (R127 and R145)
- Glutamate (E270)
- (Auld 2004)
- The zinc component is essential for activity
- Cysteine, sulfides, and cyanide, but not diisopropylphosphofluoridate (DFP) or phenylmethanesulfonyl fluoride (PMSF)
- Metal ions and anions (Geoghegan et al. 1983b)
- Strongly inhibited by the chelating agent 1,10-phenanthroline
- Ochratoxin A is a competitive inhibitor (Pitout and Nel 1969)
- Phosphonates (Kaplan and Bartlett 1991)
- Latexin (Normant et al. 1995)