For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Lactate Dehydrogenase - Manual
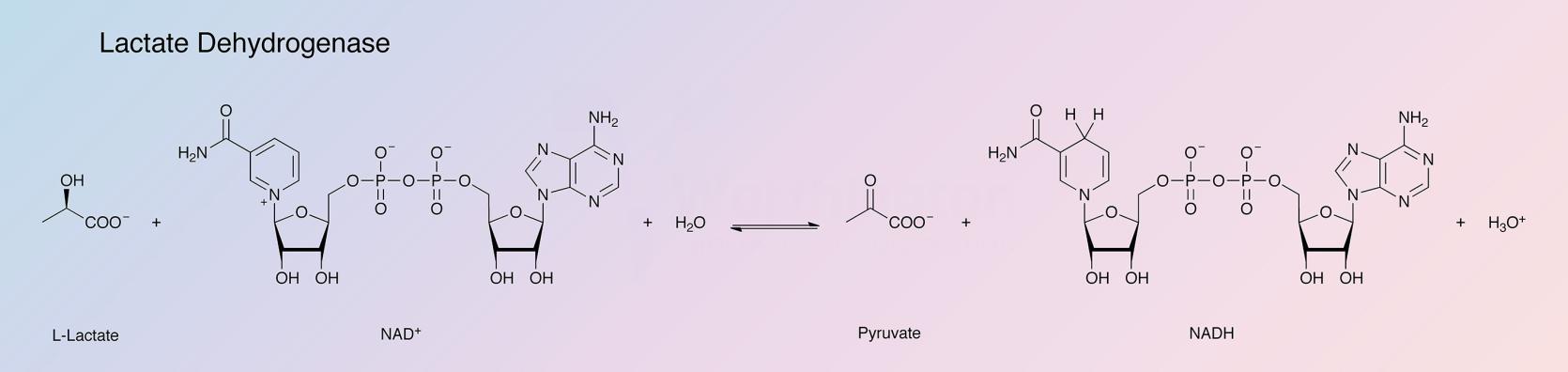
Lactate dehydrogenase (LDH) catalyzes the following reaction:
![]()
Human LDH has been reported on by Ringoir and Plum (1975), Emes et al. (1974), Markel and Janich (1974), McQueen (1974), Burd and Usatequi-Gomez (1973), and McKee et al. (1972); that from pig by Hinz and Jaenicke (1975), Bloxham et al. (1975), Chen and Engel (1975), Eventoff et al. (1974), Jaenicke (1974), Whitaker et al. (1974), Holbrook and Ingram (1973), Holbrook and Stinson (1973), and Stinson and Holbrook (1973). Adams et al. (1973) and Taylor et al. (1973) have reported on dogfish LDH, and Carlotti et al. (1974) and Ryan and Vestling (1974) on that of rat liver and hepatomas. Fritz et al. (1973) report on different rates of tissue turnover of the rat isozymes. Kabura and Konvich (1972) extracted LDH isozymes from mouse brain. Ehmann and Hultin (1973) studied chicken breast LDH M5. Eby et al. (1973) report on frog LDH and Lim et al. (1975) on that from salmonid fish. Long and Kaplan (1973) report on horseshoe crab and sea worm LDH. That from potatoes has been studied by Rothe (1974) and Davies and Davies (1972). Brown et al. (1975) and Allsopp and Matthews (1975) report on the Actinomyces and Mycoplasma enzymes.
Mammalian lactate dehydrogenase (LDH) exists as five tetrameric isozymes composed of combinations of two different subunits. The isozymes differ in catalytic, physical and immunological properties. Cahn et al. (1962) refer to the polypeptide subunits as "H" and "M", which combine to form two pure types of isozymes, H4 and M4, and three hybrids, H3M, H2M2, and HM3. Type H4 is the most negatively charged at pH 7 and in zone electrophoresis appears nearest the anode. Subunit "H" predominates in heart muscle LDH which is geared for aerobic oxidation of pyruvate. The "M" subunit predominates in skeletal muscle and liver and is concerned more with anaerobic metabolism and pyruvate reduction (Fritz 1965).
A sperm isozyme (isozyme x) has been characterized from testes and spermatozoa (Zinkham et al. 1964; Stambaugh and Buckley 1967). McKee et al. (1972) indicate there to be several. LDH-X differs immunologically and enzymatically from LDH 1-5. (Spielman et al. 1973; Goldberg 1972).
LDH isozymes in the developing fetus have been reported on by Werthamer et al. (1973) and their variations with age by Gerlach and Fegler (1973). See also Ringoir and Plum (1975), Mitsutaka (1974), O'Carra et al. (1974), Glass and Doyle (1972), and Wilkinson and Walter (1972). Silverstein and Boyer (1964) compared kinetics of beef heart and rabbit muscle LDH.
LDH is of interest clinically in that the serum level of certain isozymes reflects pathological condition in particular tissues.
Studies on structure, binding sites and kinetics include the following: Adams et al. (1973), Bartholmes et al. (1973), Bishop et al. (1972), Bloxham et al. (1975), Cho and Swaisgood (1974), Dudman and Zerner (1973), Ehmann and Hultin (1973), Hinz and Jaenicke (1975), Holbrook and Ingram (1973), Holbrook and Stinson (1973), Jaenicke (1974), Levetzki (1972), Low et al. (1973), Millar (1974), Mitsutaka (1974), Saito (1972), Stinson and Holbrook (1973), Tienhara and Meany (1973). Whitaker et al. (1974) report on immobilized LDH.
Characteristics of LDH from Beef Heart:
The enzyme is specific for L(+)lactate. Meister (1950) reports it reduces several α-keto and α,β-diketo acids but at about one-tenth the rate of reduction of pyruvate.
Vallee and Williams (1975) have reported on its subunit dissociation at low pH. See also Yang and Schwert (1972) and Gold and Segal (1965).
35,000/subunit (Fosmire and Timasheff 1972). 136,700 ± 2,100/tetramer (Huston et al. 1972).
![]() =14.9.
=14.9.
A number of organic compounds which stabilize the enzyme, such as dimethyl sulfoxide, ethanol, and methanol, are reported by George et al. (1969). Diethylstilbestrol and several of its derivatives also stabilize the enzyme (Cohen et al. 1969).
Characteristics of LDH from Rabbit Muscle:
Molecular Weight
140,000
Extinction Coefficient
![]() =8.8
=8.8
Lovell and Winzor (1974) report that the tetramer dissociates completely into two dimers (molecular weight 70,000) in acetate-chloride buffer pH 5 (conditions without effect on beef heart LDH). Phosphate and pyridine nucleotides stabilize the quarternary structure of the tetramer. Phosphate has an activation effect. See also Cho and Swainsgood (1973).
LDH is quite stable. It is inactivated by iodide. Inhibition by p-mercuribenzoate is slow. See Schwert and Winer (1963); also Anderson et al. (1974) and Bloxham et al. (1975).
See Borgmann et al. (1974).
Reaction kinetics have been reported by Stambaugh and Post (1966) and Zewe and Fromm (1965).