For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Cellulase - Manual
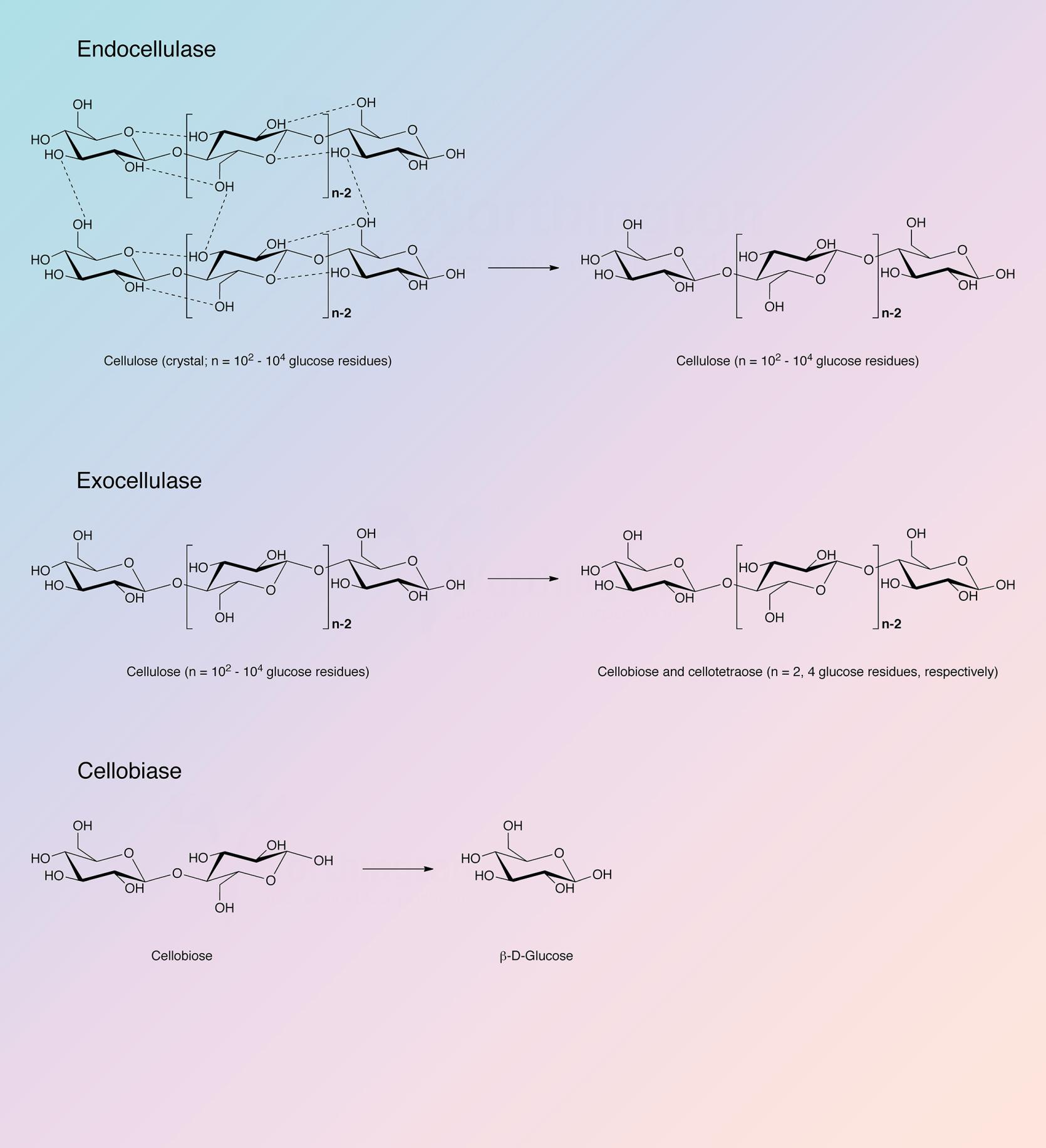
Cellulase refers to a group of enzymes which, acting together, hydrolyze cellulose.
Cellulose is a linear polysaccharide of glucose residues connected by β-1,4 linkages. Like chitin it is not cross-linked. Native crystalline cellulose is insoluble and occurs as fibers of densely packed, hydrogen bonded, anhydroglucose chains of 15 to 10,000 glucose units. Its density and complexity make it very resistant to hydrolysis without preliminary chemical or mechanical degradation or swelling. In nature cellulose is usually associated with other polysaccharides such as xylan or lignin. It is the skeletal basis of plant cell walls. Cellulose is the most abundant organic source of food, fuel and chemicals (Spano et al. 1975). However, its usefulness is dependent upon its hydrolysis to glucose. Acid and high temperature degradation are unsatisfactory in that the resulting sugars are decomposed; enzymatic degradation (cellulase) is the most effective means of degrading cellulose into useful components. Although cellulases are distributed throughout the biosphere, they are most prevalent in fungal and microbial sources.
The Cullulase Complex from Trichoderma reesei:
The Trichoderma reesei complex is a true cellulase in the most rigid sense, being able to convert crystalline, amorphous, and chemically derived celluloses quantitatively to glucose. It has been established that: a) the system is multi-enzymatic; b) that at least three enzyme components are both physically and enzymatically distinct; and c) that all three components play essential roles in the overall process of converting cellulose to glucose (King and Vessal 1969).
Reese et al. (1950) first proposed the enzyme mechanisms involved in the degradation of cellulose. At least two steps are involved: first, a prehydrolytic step wherein anhydroglucose chains are swollen or hydrated and secondly, hydrolytic cleavage of the now susceptible polymers either randomly or endwise.
Reese and Levinson made an extensive comparative study of the action of cellulolytic organisms showing many cellulolytic organisms degrade modified cellulose, but often do not show corresponding activity on native cellulose (Reese and Levinson 1952). Mandels and Reese first described the cellulase complex from Trichoderma viride, later named Trichoderma reesei, from which Worthington cellulase is produced (Mandels and Reese 1957).
The original Worthington assay was developed in our laboratory in 1957 and used carboxymethylcellulose, unaware that a test using this substrate had been described by Levinson and Reese in 1950 (Levinson and Reese 1950). Today’s Worthington assay uses the rate of glucose formation, a technique first described in 1960 (Meyers et al. 1960).
The cellulase complex of T. reesei has been most thoroughly studied. It is complete in that it can convert native cellulose as well as derived celluloses to glucose (King and Nessal 1969). Howell and Stuck (1975) have described the complex and indicate it to be remarkably resistant to inhibitors except carbohydrates, particularly cellobiose and excess cellulose. Okada (1975) obtained a number of active fractions from T. reesei differing in carbohydrate content and specificity but otherwise identical.
Berghem and Pettersson (1973) reported on the T. reesei enzyme involved in the first step of cellolose degratation, a β-1,4-glucan cellobiohydrolase which acts upon crystalline cellulose. Cellobiose is the principle product. The enzyme was further characterized by Berghem, Pettersson and Axio-Fredriksson (1975). They indicated a molecular weight of 42,000 based on amino acid and carbohydrate analysis. The enzyme contained 9.2% carbohydrate covalently bound. The pH optimum is about 4.8 and the reaction rate can be enhanced by addition of cellobiase and additional endo-glucanase. The identification of the genes for the other cellulase and hemicellulase enzymes of T. reesei has been completed, and the characterization of many of these enzymes has also been achieved (Kubicek 2011).
Recently researchers have been investigating the genomes of mutant species to correlate cellulase activity with specific gene sequences (Le Crom et al. 2009, and Vitikainen et al. 2010). Current research has explored the use of cellulase in the production of biofuels and biorefinery products (Kubicek 2012).
Cellobiohydrolase I (Cel7A) and cellobiohydrolase II (Cel6A) are the predominant cellulase proteins in all major commercial preparations. The endoglucanases and hemicellulases represent less than 10% of the extracellular extract (Chundawat et al. 2011).
The cellulase complex from Trichoderma reesei contains endoglucanases, cellobiohydrolases, and beta-glucosidases. Information concerning the known sequences of these indivudual enzymes have been organized in the following table. Each individual enzyme functions as a monomer.
The T. reesei genome contains 9143 genes, with a surpsingly small number of cellulase and hemicellulase genes as compared to other Trichoderma species. T. reesei contains a total of 200 glycosyl-hydrolase (GH) encoding genes, several of which share homology with bacteria suggesting they may have been obtained by horizontal gene transfer (Kubicek 2013). 41% of the GH and other carbohydrate active genes were reported to occur in 25 discreet clusters (Martinez et al. 2008). Several of these clusters also contain genes encoding non-ribosomal peptide and polyketide synthases suggesting these genes are important for the ability of Trichoderma to compete in its natural environment (Kubicek 2013). Production of cellulases is induced by the presence of cellulose, lactose, or sophorse; the enzyme complexes are not formed constitutively. Expression is controlled by a variety of positive and negative transcription factors (Kubicek et al. 2009).
- Digestive tablets
- Removal or softening of cellulose in food preparation (Toyama 1963)
- Protoplast preparation from plants (Cocking 1960)
- Various manufacturing processes (White and White 1997)
|
|
Accession Number |
Molecular Weight |
|
Endoglucanase I |
P07981 |
46.0 kDa |
|
Endoglucanase II |
P07982 |
42.2 kDa |
|
Endoglucanase IV |
O14405 |
33.4 kDa |
|
Endoglucanase V |
P43317 |
22.8 kDa |
|
Endoglucanase VII |
Q7Z9M7 |
25.1 kDa |
|
beta-1,4-glucanase |
O00095 |
23.5 kDa |
|
Cellobiohydrolase I |
P62694 |
52.2 kDa |
|
Cellobiohydrolase II |
P07987 |
47.2 kDa |
|
beta-Glucosidase I |
AAA18473 |
75.3 kDa |
|
beta-Glucosidase II |
O93785 |
52.1 kDa |
|
|
Class |
Architecture |
Topology |
|
Endoglucanase I |
Mainly beta |
Distorted sandwich |
1,4-Beta-D-Glucan Cellobiohydrolase |
|
Endoglucanase II |
Alpha Beta |
Alpha-Beta Barrel |
TIM Barrel |
|
beta-1,4-glucanase |
Mainly beta |
Sandwich |
Jelly Rolls |
|
Cellobiohydrolase I |
Mainly beta |
Distorted Sandwich |
1,4-Beta-D-Glucan Cellobiohydrolase I; Chain A |
|
beta-Glucosidase I and II |
Alpha Beta |
Alpha-Beta Barrel |
TIM Barrel |
Varies with the substrate in the range 4.2 - 5.2.
- 4.5-7.2 (Theoretical)
- Nonionic detergents like Triton X-100 (White and White 1997)
- Carbohydrates, particularly cellobiose and excess cellulose (Howell and Stuck 1975)