For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Neutral Protease (Dispase) - Manual
Neutral protease is an extremely stable Zn-metalloendopeptidase that is produced by Paenibacillus polymyxa. It is involved in the generation of beta- and alpha-amylases from the large amylase precursor. Although related to trypsin, neutral protease is significantly less harmful to cells and can help prevent unwanted cell clumping without cell membrane damage after one hour incubations (Alvarez et al. 2006).
In the 1950s neutral protease was first crystallized and characterized from Bacillus subtilis and was thought to be an extracellular product (Fukumoto and Negoro 1951, Guntelburg 1954, Ottesen and Spector 1960).
Into the 1970s the proteases of Bacillus thermoproteolyticus (Keay et al. 1970), Bacillus megaterium (Millet et al. 1969), Bacillus cereus (Feder et al. 1971), Streptomyces griseus (Nomoto et al. 1959), Aspergillus oryzae (Misaki et al. 1970), and Serratia (Miyata 1971) were preliminarily studied. Subsequently, Griffin and Fogarty investigated the proteolytic and starch degrading enzymes of Bacillus polymyxa (Griffin and Fogarty 1971 and 1973), and reported on the effects of different factors influencing the proteolytic activities.
Work done in the early 1980s highlighted the use of neutral protease as a means of separating epidermal sheets (Kitano and Okada 1983).
In 1993 Ash et al. (1991 and 1993) compared the 16S rRNA gene sequences of different Bacillus species and defined a new genus named Paenibacillus, to which the former Bacillus polymyxa now belongs to as Paenibacillus polymyxa.
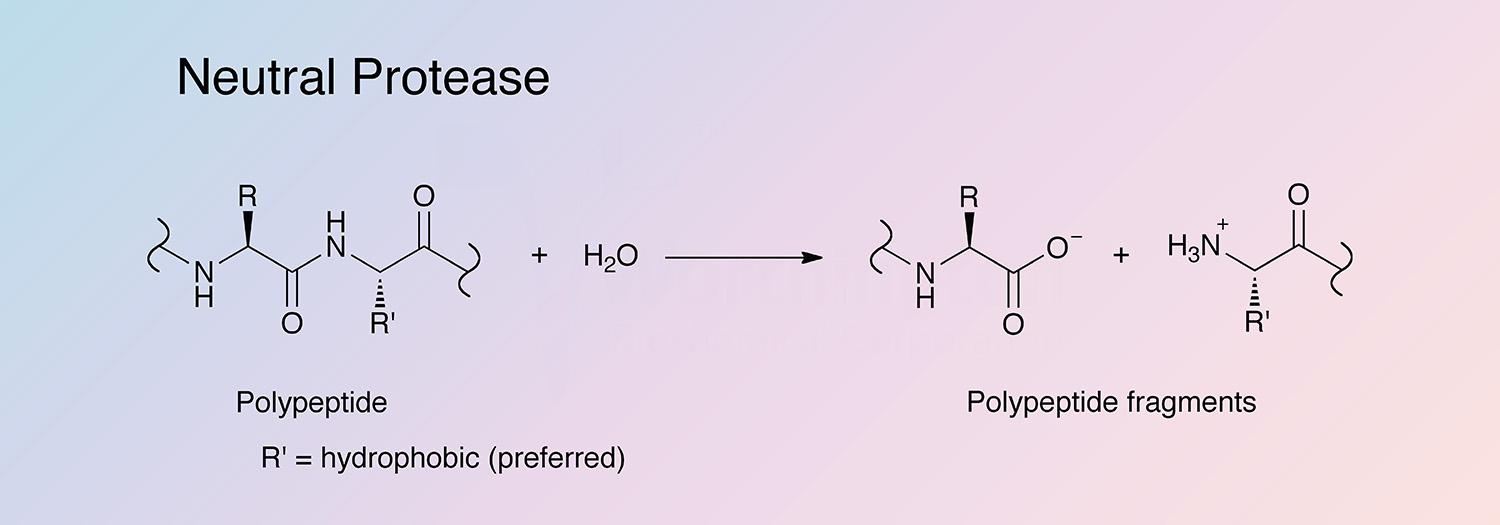
Neutral protease is a non-specific metalloprotease. It cleaves fibronectin, collagen IV, and to a lesser extent collagen I, but it does not cleave collagen V or laminin. It hydrolyzes N-terminal peptide bonds of non-polar amino acid residues and may preferentially attack denatured and intercellular proteins with exposed hydrophobic amino acid residues. It is believed to bind one zinc ion and four calcium ions per subunit. Unlike other Bacillus species that produce neutral, alkaline, or a mixture of both proteases, Paenibacillus polymyxa is one of three species that produces only a neutral protease (Fogarty and Griffin 1973).
The enzyme is known to contain 1g-atom of zinc per g-mol of purified enzyme. If this zinc component is removed by chelating agents such as EDTA or EGTA, an inactive apoenzyme is obtained. Calcium has been detected in the purified protein and is believed to play a role in maintaining the structure and configuration, and preventing autolysis (Griffin and Fogarty 1973 and Alvarez 2006).
Neutral protease is produced by the extracellular neutral protease gene, npr (GenBank accession number BAA00734), which shares 62% identity with that of Bacillus subtilis (Koide et al. 1986). A single 978 bp open reading frame is present and the presence of a pro sequence is common to all secreted proteases of Bacilli. (Takekawa et al. 1991). The Npr sequence from Asn-287 to Gly-590 shows considerable homology with sequences of neutral proteases such as thermolysin (55% identical) from Bacillus thermoproteolyticus.
- Tissue disaggregation and subcultivation
- Prevention of unwanted cell clumping
- Preparation of cells for culture
- Separation of intact epidermis from dermis and intact epithelial sheet in culture from the substratum (Kurt et al. 1989)
- Harvest and transfer of normal, diploid cells and cell lines (Matsumura et. al 1975)
- Gentle and intact detachment of epidermal cells (Kitano and Okada 1983)
P29148
- Class: The protein consists of two distinct domains: the C-terminal is mainly alpha, while the N-terminal is mainly beta.
- Architecture: The distinct architectures of the C-terminal and N-terminal are orthogonal bundle and roll, respectively.
- Topology: The C-terminal domain is elastase-like and the N-terminal domain is neutral protease-like.
32.5 kDa
5.9-7.0 (Fogarty and Griffin 1973)
5.14 (Theoretical)
- 45,350 cm-1 M-1 (Theoretical)
- E1%,280 = 13.96 (Theoretical)
- Glutamic Acid (E424)
- Histidine (H507)
- Ca2+, Mg2+, Mn2+, Fe2+, and Al3+
Manganese has a greater activating effect in the case of Paenibacillus polymyxa than other Bacilli neutral proteases (Griffin and Fogarty 1973).
- EDTA, EGTA, Hg2+
- Other heavy metals (Griffin and Fogarty 1971)
- Not serum