For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Clostripain (Endoproteinase-Arg-C) - Manual
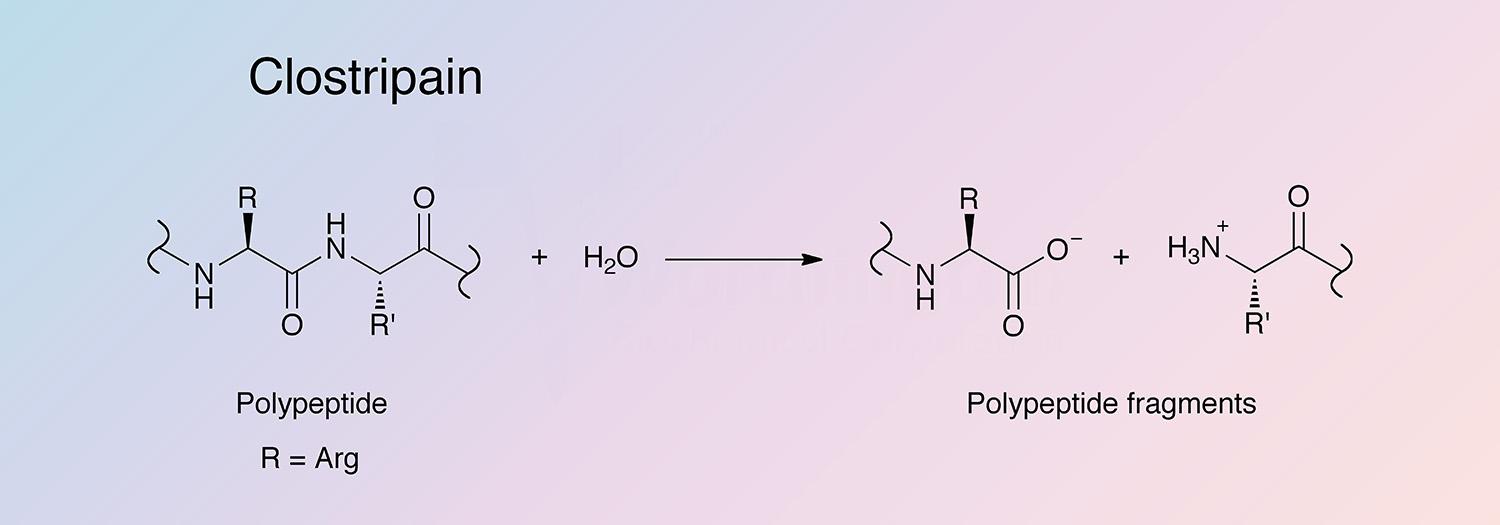
Clostripain is a cysteine-activated protease found, along with collagenase and other proteases, in culture filtrates of Clostridium histolyticum. It is unique in its specificity for the carboxyl peptide bond of arginine and its dependence on thiol and calcium ions.
The bacteria from which clostripain is purified first gained attention during World War I because of its severe consequences to the wounded (Mitchell and Harrington 1971). Cl. hisolyticum is only one of the organisms with consequential pathogenic properties, but its proteolytic activity in cell-free culture filtrates gained attention as far back as 1917 (Weinberg and Ségun 1917, and Mitchell and Harrington 1971).
In 1931, digestion of horse tendons was described by Weinberg and Randin. A year later they identified an exotoxin, which they termed “ferment fibrinolytique”, as the cause of digestion (Weinberg and Randin 1932). It was later found that this digestion was caused by a variety of proteolytic enzymes, including a cysteine-activated proteinase, clostripain (Kochalaty and Weil 1938, and Maschmann 1938).
In 1948, Kochalaty and Krejci first successfully isolated clostripain in relatively pure form (Mitchell and Harrington 1968). Ogle and Tytell refined the purification technique in 1953 and first reported on its specificity (Ogle and Tytell 1953).
When the narrow substrate specificity of clostripain became of interest, confusion existed in the literature regarding the identity of clostripain (Mitchell and Harrington 1971). Prior to its description as clostripain by Labouesse and Gros in 1960, and later Mitchell and Harrington in 1968, it was referred to as g-protease (Bard and McClung 1948, and Oakley and Warrack 1950), amidase-esterase (Nordwig and Strauch 1963), and clostridiopeptidase B (Mitchell and Harrington 1968).
Recent work with clostripain has included cell isolation and its use as a model target of protease inhibitors for the treatment of clostridial infections (Wang et al. 2004, and Gusman et al. 2001).
Clostripain selectively hydrolyzes arginyl bonds and lysyl bonds at a lower rate. It can also act as a transpeptidase with maximal activity at pH 7.6-9.0 (Anderson 1985, and Fortier and MacKenzie 1986).
Clostripain is a heterodimer. The mature chain is composed of 526 residues. The two chains are held together by strong noncovalent forces (Gilles et al. 1979, and Ullman and Bordusa 2004). The catalytic sulfhydryl residue of the active site is believed to be Cys41 (heavy chain residue). The precursor contains a 27 amino acid putative signal peptide, a 23 amino acid propeptide, a 131 amino acid light chain subunit, a 9 amino acid linker peptide, and a 336 amino acid heavy chain subunit (Ullman and Bordusa 2004).
Both the heavy and light chains are encoded by a single gene with a 1581 nucleotide open reading frame (ORF). Upon expression of the gene, the entire ORF (the signal region, proregion, and 9 amino acid peptide linker) is transcribed. Postranslational processing produces the heterodimeric active enzyme (Dargatz et al. 1993).
- Peptide mapping
- Sequence analysis
- Cell isolation (Wang et al. 2004)
- Hydrolysis/condensation of amide bonds
- Peptide synthesis (Meiwes et al. 1991)
P09870
- 53.0 kDa (Theoretical)
- Light chain: 12.5 kDa, Heavy chain: 45 kDa (Gilles et al. 1979)
- 7.4-7.8 (activity against a-benzoyl-arginine ethyl ester) (Mitchell and Harrington 1968)
4.8-4.9 (Mitchell and Harrington 1971)
- 87,890 cm-1 M-1 (Theoretical)
- E1%,280= 16.57 (Theoretical)
- Cysteine (C41, heavy chain)
- Sulfhydryl requirement: dithiothreitol, cysteine, or other reducing agents
- Calcium ion is essential
- Reducing agents
- EDTA
- Oxidizing agents
- Sulfhydryl reagents (such as TLCK) (Porter et al. 1971)
- Co2+, Cu2+, Cd2+, and heavy metal ions
- Citrate, borate and Tris anions partially inhibit